Metrohm tiamo 2.1 (ProcessLab) User Manual
Page 871
■■■■■■■■■■■■■■■■■■■■■■
5 Method
tiamo 2.1 (ProcessLab)
■■■■■■■■
855
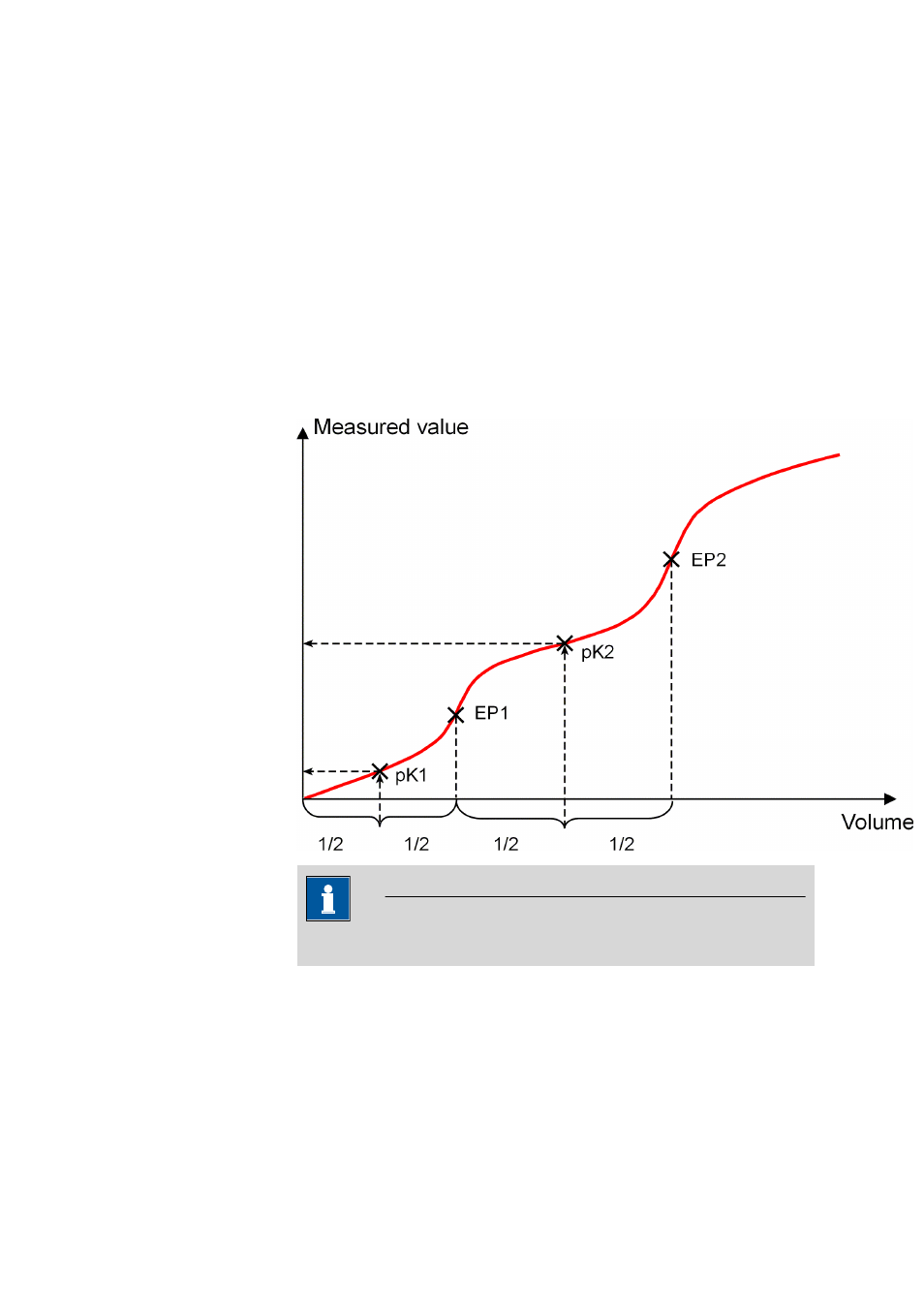
If the activities of the acid and the conjugated base are equal (a
A
= a
B
), then
pH=pK
a
. This is the value at the half neutralization point and can be
extrapolated from the titration curve. A careful pH calibration is necessary
for pK evaluations. Nonetheless, the determined pK value is only an approx-
imation, as the ionic strengths are not taken into account. In order to obtain
more accurate values, titrations must be carried out with decreasing ionic
strengths and the results extrapolated to the ionic strength zero. pK evalu-
ation in aqueous solution is limited to the range 3.5 < pK < 10.5 because of
the leveling effect of strong acids and the lack of jumps with very weak acids.
pK values of mixtures of acids and polyvalent acids can also be determined.
In non-aqueous solutions the half neutralization potential (HNP) is fre-
quently used instead of the pK value. The HNP is evaluated in the same way
as the pK value.
Note
If a start volume is to be added then it must be smaller than 1/2 V(EP1).
- 915 KF Ti-Touch (382 pages)
- 800 Dosino (53 pages)
- 767 Calibrated Reference (23 pages)
- 940 Professional IC Vario ONE/SeS/Prep 2 (54 pages)
- 754 Dialysis Unit (49 pages)
- 815 Robotic Soliprep for LC (76 pages)
- Vision Manual (207 pages)
- tiamo 2.1 Manual (1532 pages)
- 825 Lab Link (37 pages)
- 808 Titrando (70 pages)
- 902 Titrando (52 pages)
- 756 KF Coulometer (163 pages)
- 756 KF Coulometer (162 pages)
- 940 Professional IC Vario ONE/LPG (98 pages)
- 850 Professional IC Anion MCS Prep 3 (154 pages)
- 850 Professional IC Anion MCS Prep 3 (152 pages)
- 904 Titrando (58 pages)
- 850 Professional IC Anion MSM-HC MCS Prep 2 (150 pages)
- 930 Compact IC Flex Oven/ChS/Deg (47 pages)
- 872 Extension Module Liquid handling (64 pages)
- 814 USB Sample Processor (91 pages)
- 814 USB Sample Processor (90 pages)
- 940 Professional IC Vario (43 pages)
- Vision – Tutorial (40 pages)
- 799 GPT Titrino (242 pages)
- 889 IC Sample Center (68 pages)
- 761 Compact IC (228 pages)
- 851 Titrando (100 pages)
- 748 DH Sample Changer (32 pages)
- 940 Professional IC Vario ONE/SeS/HPG (51 pages)
- 896 Professional Detector – Amperometry (62 pages)
- 877 Titrino plus (139 pages)
- 881 Compact IC pro – Anion (129 pages)
- 940 Professional IC Vario ONE/ChS/HPG (112 pages)
- 930 Compact IC Flex Deg (41 pages)
- 840 PC Control 5.0 / Touch Control (351 pages)
- 940 Professional IC Vario ONE/Prep 1 (45 pages)
- 776 Dosimat (42 pages)
- 717 Sample Changer (36 pages)
- 815 Robotic USB Sample Processor XL (114 pages)
- 815 Robotic USB Sample Processor XL (113 pages)
- 940 Professional IC Vario ONE/SeS/PP (126 pages)
- 838 Advanced Sample Processor Installation Instructions (109 pages)
- 700 Dosino (55 pages)
- 719 S Titrino (152 pages)
