Results not reproducible – Metrohm 797 VA Computrace User Manual
Page 286
9 Troubleshooting
797 VA Computrace – Software
274
If the overlapping is too large, the peak can no longer be evalua-
ted. In this case chemical or measurement technique countermea-
sures must be used to attempt to separate these peaks better. Pos-
sible measures include changing the pH value, changing the sup-
porting electrolyte concentration, changing the supporting elect-
rolyte, use of complexing agents
(see Complex formation, section
9.6),
longer
deposition
times and modifying or changing the
measurement technique.
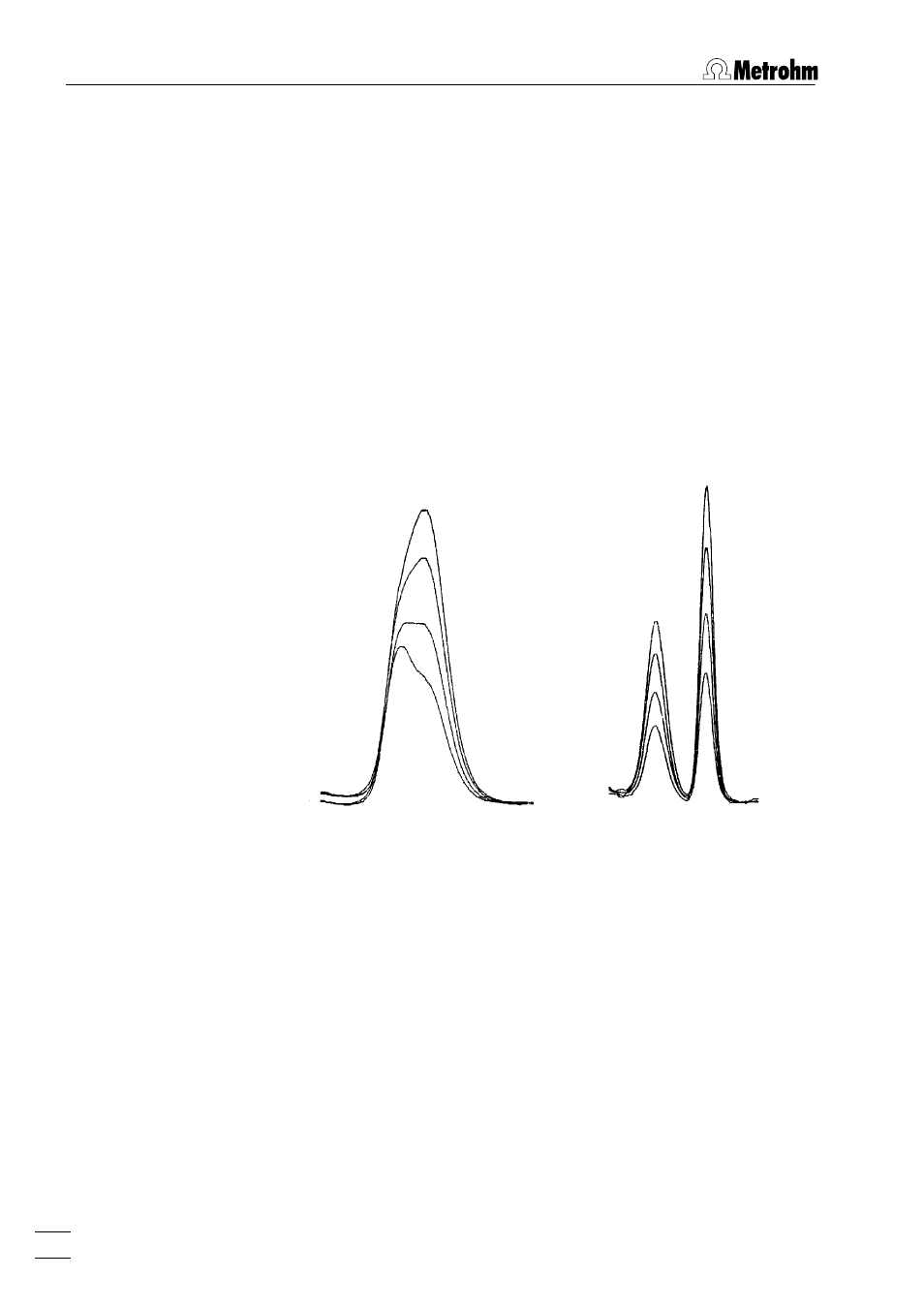
• Determination of lead and thallium
With a supporting electrolyte of pH = 1, Pb and Tl peaks over-
lap greatly. Changing the pH to 13 separates the two peaks.
(The separation of lead and thallium can also be achieved by
subsequent electrolysis or in acetate buffer with EDTA).
Supporting electrolyte:
Supporting electrolyte:
pH = 1
pH = 13
Analysis solution:
0.5 mg/L Pb; 1 mg/L Tl
Standard addition:
with 10 μg Pb and 10 μg Tl
Electrode: SMDE
Calibration with chemically non-isoformal standards
With all possible
Calibration
techniques, it must be ensured that the
standards used for calibration are chemically isoformal with the
analytes. The standard substances must therefore have the same
valency (e.g. with Fe, Al) or complex formation form (e.g. with As,
Cr, Se) as the substances already present in the analysis solution. If
this is not the case the calibration can produce completely wrong
results owing to the different peak voltages and sensitivities.
Results not reproducible
Voltammetric measurements (including CVS and CPVS!) strongly
depend on the temperature.
Pb
Tl
