Complex formation – Metrohm 797 VA Computrace User Manual
Page 284
9 Troubleshooting
797 VA Computrace – Software
272
Analysis solution:
deionised water
Standard addition:
with 50 ng Zn
Electrode:
HMDE (enrichment 60 s at –1.2 V)
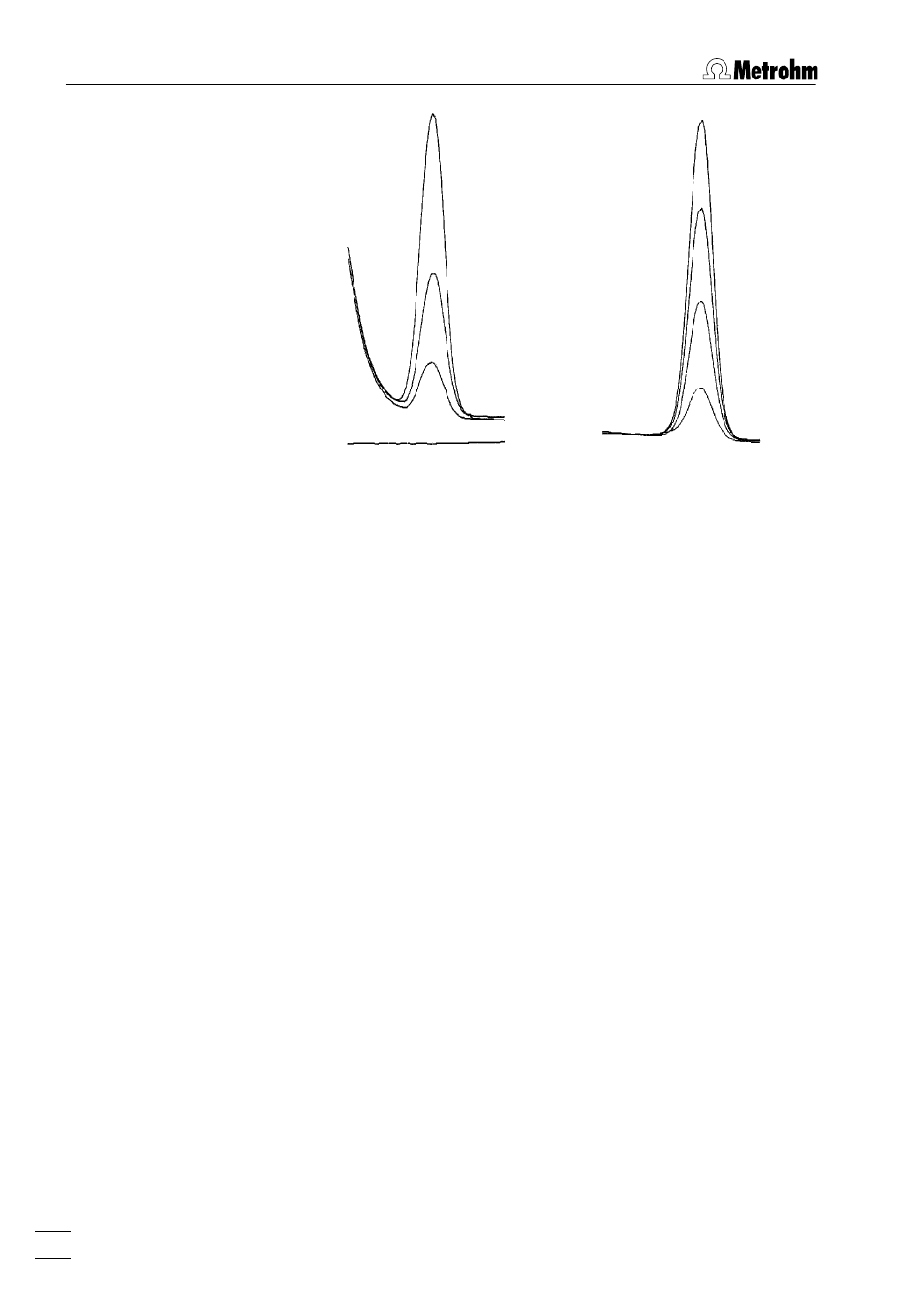
Complex formation
Substances determined polarographically can occur in various com-
plexed forms, depending on the composition of the analysis solu-
tion. As complexing is always associated with a shift in the half-
wave potential and the limiting current, difficulties can arise in the
peak evaluation. Such difficulties must be eliminated by appropriate
changes in the composition of the supporting electrolyte.
If it is not possible to remove the interfering complexing agents
from the analysis solutions or to mask them by suitable substances,
it is often helpful to change the pH of the supporting electrolyte.
Another measure that is often used involves the addition of a
ligand of high complexing power (e.g. EDTA)
to bring about 100%
change of the analyte to a definitive form. The latter possibility is
also used in the following example:
• Copper determination in chloride-containing solutions
Copper can occur in chloride-containing solutions as both a
CuCl
4
2–
and a CuCl
2
–
complex. The two associated current
peaks are near each other. In unfavourable cases, the determi-
nation of copper is not possible. The difficulties disappear after
addition of the complexing agent EDTA as now all copper is
completely in the form of a Cu-EDTA complex. (Increasing the
chloride concentration [e.g. by addition of 1 mL of a 1.5 mol/L
KCl solution of the greatest possible purity per 10 mL analysis
solution] would also give a clearly defined current peak for
CuCl
2
–
.)
Supporting electrolyte:
Supporting electrolyte:
without EDTA
with EDTA (0.001 mol/L)
