Creating/editing a device record or template – Fluke Biomedical 601PRO XL User Manual
Page 87
A U T O / S T E P M O D E S
4 - 7
3. Creating/Editing a Device Record or Template
☛
Note:
In this section, details about device records are given to explain how to
create, edit, delete, and print them, as well as transmit and receive them
from a computer. These details also apply to templates with minor
differences that are explained in chapter 2, Configuring for Device
Records or Templates. If
templates
was selected under
System Setup
,
then replace Device Records with Templates in the following display
examples.
Device records and templates require the following information at a minimum: the
test standard
, the
class/type
, the
protective earth resistance current
, and the
number of applied parts
. In addition, Device Records also require the
control
number
, and Templates require a name.
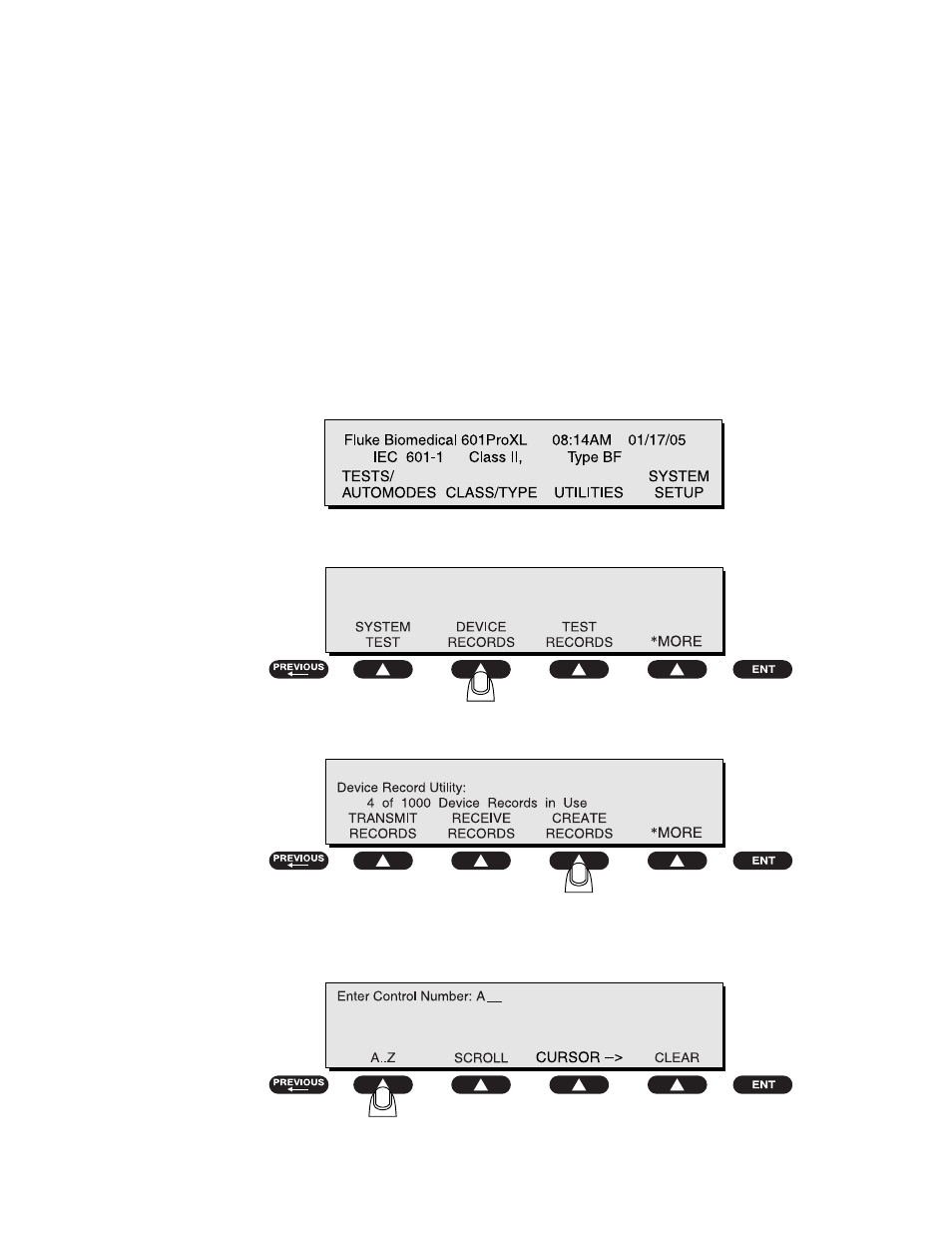
• At the
MAIN MENU
, press
UTILITIES
:
baw004f.eps
• Press
DEVICE RECORDS
.
baw081f.eps
• At the
Device Record Utility
menu, press
CREATE RECORD
.
baw082f.eps
• Enter a
control number using the SOFT KEYS
and the
test-shortcut
keys, and press
ENT
:
baw083f.eps
