Merit Medical HepaSphere Microspheres(With Doxorubicin) IFU-Int'l User Manual
Page 6
• Two methods for embolic aliquot sequestering for injection may be used:
- Option 1: Connect a 3 way-stopcock to the 30ml syringe containing
the doxorubicin loaded HepaSphere Microspheres to the infusion micro
catheter and use a 1ml syringe for injection through the open port of the
3 way-stopcock.
- Option 2: Serial aliquots of the doxorubicin loaded HepaSphere
Microspheres can be drawn from the 30ml syringe into a 1ml injection
syringe through a 3 way-stop cock that is not attached to the infusion
catheter. The 1ml syringe containing each aliquot can be attached
independently to the infusion microcatheter and injected.
• Invert the 30ml syringe back and forth to maintain the homogenous
suspension of the HepaSphere Microspheres mixture.
• Under continuous fluoroscopic guidance, inject the aliquot of
doxorubicin loaded HepaSphere Microspheres in a slow, non forceful,
pulsatile manner over a time period of approximately 1 minute per ml of
microspheres solution. Always inject under free-flow conditions and
monitor for reflux.
Note: Reflux of embolic spheres can induce immediate ischemia of
untargeted tissues and vessels.
• When stasis in the feeding pedicle occurs while delivering the
doxorubicin HCl loaded HepaSphere Microspheres, wait a minimum of 5
minutes then perform a selective angiogram after the full 5 minutes wait
to verify the cessation of antegrade flow.
• If cessation of antegrade flow has not occurred, continue infusion
under fluoroscopic guidance until the desired devascularization is
obtained.
• After the HepaSphere Microsphere infusion is completed, remove the
catheter while maintaining gentle aspiration to avoid dislodging any
residual HepaSphere Microspheres that may still be in the catheter
lumen. Discard the catheter after removal and do not reuse.
• Discard any open vial or unused HepaSphere Microspheres.
CAUTION
In the event that the catheter becomes obstructed or significant infusion
resistance is encountered during injection, do not attempt to flush the
catheter with excessive pressure because reflux of embolic material may
occur resulting in untargeted embolization. Remove the catheter while
applying gentle aspiration and discard.
CONSERVATION AND STORAGE
HepaSphere Microspheres must be stored in a dry, dark place in their
original vials and packaging. Use by the date indicated on the labels of
the outer box and pouch.
When the procedure of reconstitution is completed, store the solution of
HepaSphere Microspheres in 2 to 8°C conditions and use within 24
hours, IF not used immediately. Do not store HepaSphere Microspheres
after contrast medium has been added.
Information on packaging:
All serious or life threatening adverse events or deaths associated
with use of HepaSphere Microspheres should be reported to the
device manufacturer.
6
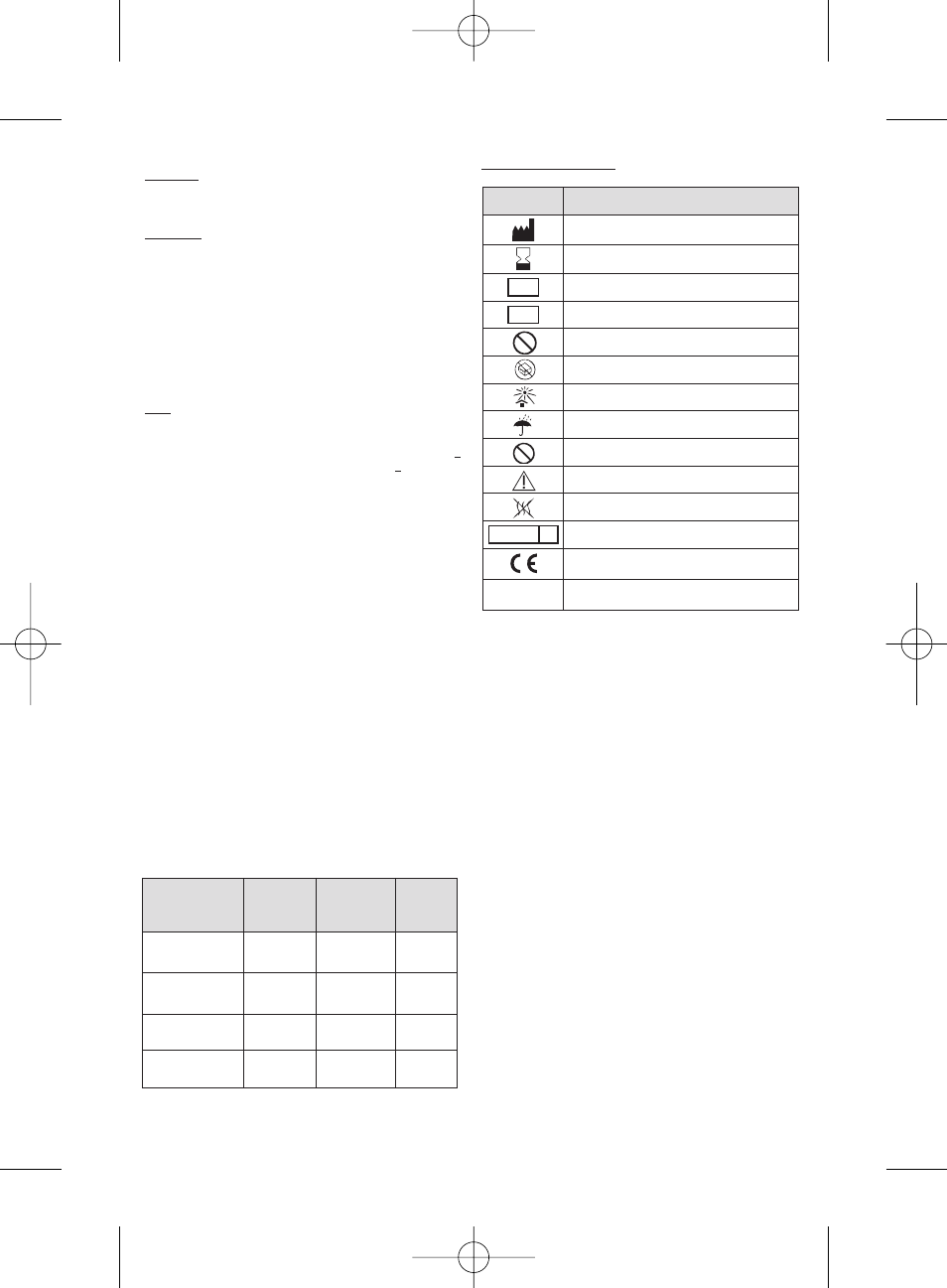
Symbol
Designation
Manufacturer: Name & Address
Use by date: year-month
LOT
Batch code
REF
Catalogue number
STERILIZE
2
Do not resterilize
Do not use if package is damaged
Keep away from sunlight
Keep dry
2
Do not re-use
Caution - Refer to Instructions For Use
Non-pyrogenic
STERILE R
Sterilized using irradiation
EC mark logo - Notified body identification : 0459
Size of dry microspheres / Size of hydrated microspheres
Size of dry products
(µm)
Colour code
(label
borders)
Quantity of
microspheres
(mg)
Reference
30-60
Orange
25
50
V 225 HS
V 250 HS
50-100
Yellow
25
50
V 325 HS
V 350 HS
100-150
Blue
25
50
V 525 HS
V 550 HS
150-200
Red
25
50
V 725 HS
V 750 HS
/
730095003_A ID 102412_IFU HS DOXO :print 9/11/12 17:07 Page 6
