Merit Medical HepaSphere Microspheres(With Doxorubicin) IFU-Int'l User Manual
Page 4
4
Contamination of the device may lead to injury, illness or death of the
patient. All procedures must be performed according to accepted aseptic
technique.
HepaSphere Microspheres MUST NOT be used in their original
dry state. They must be reconstituted before use. HepaSphere
Microspheres swell in aqueous solution. The magnitude of swelling
depends on the ionic concentration of the solution. The microspheres
swell to approximately four times their diameter in 0.9% NaCl aqueous
solution and non-ionic contrast media, as compared to their initial dry
diameter. The magnitude of swelling when loaded with doxorubicin HCl
is dependent upon the amount of drug with which the product is loaded.
Lyophilized doxorubicin HCl must be reconstituted in NaCl 0.9 % solution.
HepaSphere Microspheres undergo a slight size decrease of about 20%
when loaded with doxorubicin HCl compared to the size in pure NaCl 0.9
% aqueous solution. HepaSphere Microspheres are compressible and
can be injected easily through microcatheters. However, injection of the
HepaSphere Microspheres before they are fully expanded could result in
failure to reach the intended embolization target and possible
embolization of a larger tissue area.
Note: Maximum recommended concentration of doxorubicin HCl is
5mg/ml. Concentrations of doxorubicin HCl above 5mg/ml substantially
increase the solution viscosity and make it difficult to handle with
HepaSphere Microspheres.
Patients with known allergies to non-ionic contrast media may require
corticosteroids prior to embolization.
Additional evaluations or precautions may be necessary in managing
periprocedural care for patients with the following conditions:
• Bleeding diathesis or hypercoagulative state
• Immunocompromise
POTENTIAL COMPLICATIONS
Vascular embolization is a high-risk procedure. Complications may occur
at any time during or after the procedure, and may include, but are not
limited to, the following:
• Paralysis resulting from untargeted embolization or ischemic injury
from adjacent tissue oedema
• Undesirable reflux or passage of HepaSphere Microspheres into
normal arteries adjacent to the targeted lesion or through the lesion into
other arteries or arterial beds, such as the internal carotid artery,
pulmonary, or coronary circulation
• Pulmonary embolism due to arteriovenous shunting
• Ischemia at an undesired location, including ischemic stroke, ischemic
infarction (including myocardial infarction), and tissue necrosis
• Capillary bed occlusion and tissue damage
• Vasospasm
• Recanalisation
• Blindness, hearing loss, and loss of smell
• Foreign body reactions necessitating medical intervention
• Infection necessitating medical intervention
• Complications related to catheterization (e.g. haematoma at the site of
entry, clot formation at the tip of the catheter and subsequent
dislodgement, and nerve and/or circulatory injuries which may result in
leg injury)
• Allergic reaction to medications (e.g. analgesics)
• Allergic reaction to non-ionic contrast media or embolic material
• Vessel or lesion rupture and haemorrhage
• Death
• Additional information is found in the Warnings section
SWELLING BEHAVIOR
HepaSphere Microspheres swell during reconstitution with NaCl 0.9%
aqueous solution and non-ionic contrast media. When hydrated in 100%
NaCl 0.9% aqueous solution or non-ionic contrast medium, or 50% non-
ionic contrast and 50% NaCl 0.9% aqueous solution, HepaSphere
Microspheres swell approximately 4 times their original dry diameter in
approximately 10 minutes. For example, HepaSphere Microspheres with
a diameter of approximately 50-100 microns in their dry state will
expand to approximately 200-400 microns during reconstitution as
recommended below. Because of the inherent variability of the swelling
process, some of the HepaSphere Microspheres will be slightly outside
of this range after reconstitution, so the physician should be sure to
carefully select the size of HepaSphere Microspheres according to the
size of the target vessels at the desired level of occlusion in the
vasculature and the nature of the aqueous solution.
Note: To expand properly HepaSphere Microspheres need to be exposed
to a minimum of 10ml solution.
The magnitude of swelling when loaded with doxorubicin HCl is
dependent upon the amount of drug with which the product is loaded.
HepaSphere Microspheres undergo a slight size decrease of about 20%
when loaded with doxorubicin HCl compared to the size in pure NaCl
0.9% aqueous solution.
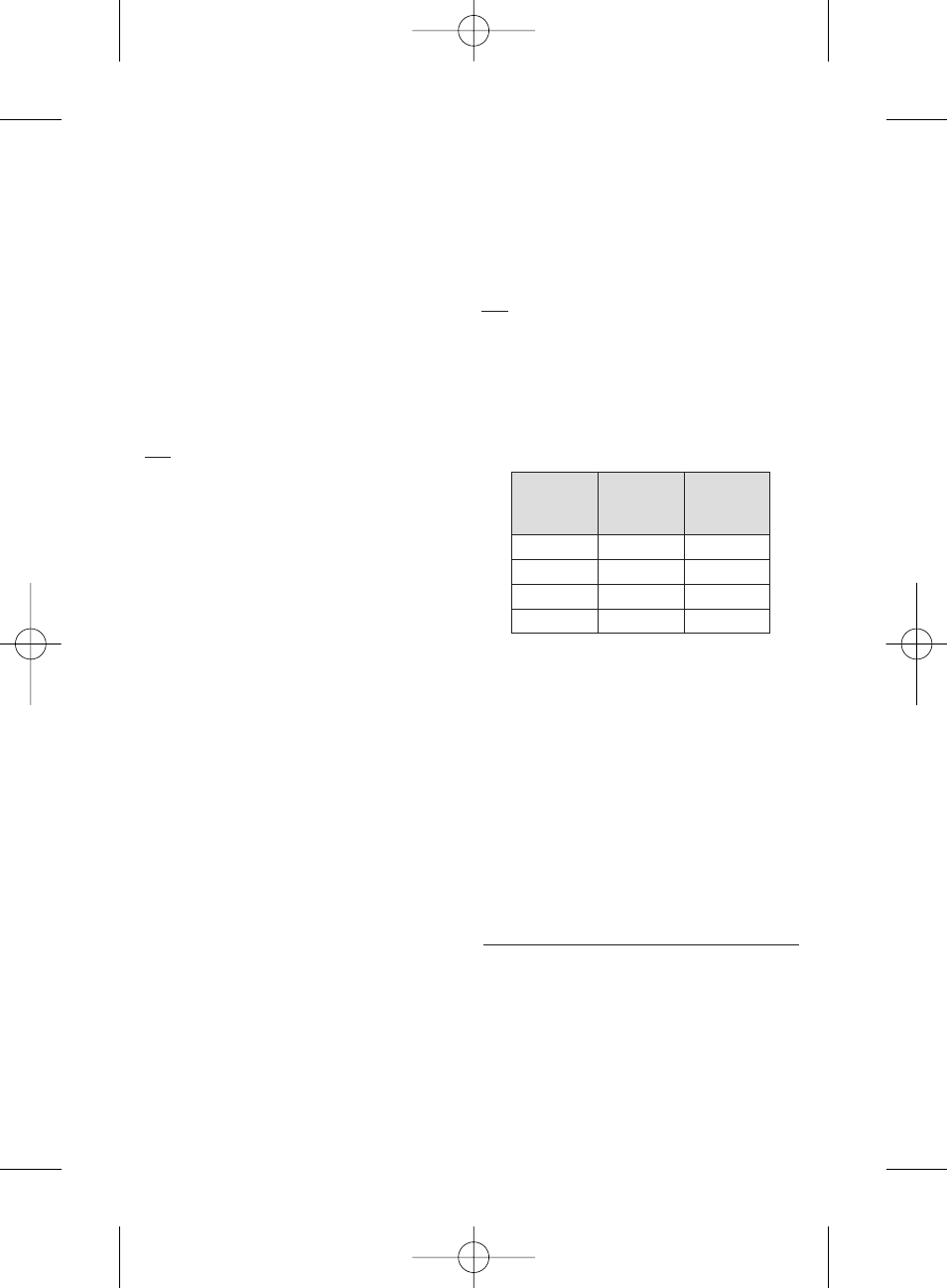
CATHETER COMPATIBILITY
HepaSphere Microspheres can be injected with microcatheters with the
following specifications:
INSTRUCTIONS
HepaSphere Microspheres must be reconstituted with 100% NaCl 0.9%
aqueous solution or non-ionic contrast medium, or 50% non-ionic
contrast medium and 50% NaCl 0.9% aqueous solution if using without
delivery of doxorubicin HCl, or loaded with doxorubicin HCl solution
before positioning the catheter.
• Carefully select the size of HepaSphere Microspheres according to the
size of the target vessels at the desired level of occlusion in the
vasculature and the nature of the aqueous solution. See the description
of “SWELLING BEHAVIOR”.
• HepaSphere Microspheres may be present outside the vial. Therefore,
the vial must be aseptically handled away from the main sterile field.
• Ensure the compatibility of the HepaSphere Microspheres with the
intended size of catheter to be used. See the table above.
• Inspect the packaging to confirm that it is intact. Remove the vial from
the pouch. The external surface of the vial is sterile.
HEPASPHERE MICROSPHERES CAN BE USED WITH OR WITHOUT
LOADING OF DOXORUBICIN HCL.
OPTION 1: PREPARATION FOR EMBOLIZATION WITHOUT
DOXORUBICIN HCL (BLAND)
The approximate reconstitution time when used without loading of
doxorubicin HCl is 10 min.
• Fill a 10ml syringe with 100% NaCl 0.9% aqueous solution or non-
ionic contrast medium (or 50% NaCl 0.9% aqueous solution and 50%
contrast). Connect the syringe to a needle of 20 gauge diameter or larger.
• To ensure proper reconstitution of the HepaSphere Microspheres,
grasp the vial horizontally in your fingertips and roll the vial several times.
This will transfer the dry contents of the vial to the sidewall.
Dry (
µm)
Approximate
Reconstituted
Size range (
µm)
Catheter Size
ID (in.)
30-60
120 - 240
≥ 0.021
50-100
200 - 400
≥ 0.021
100-150
400 - 600
≥ 0.024
150-200
600 - 800
≥ 0.027
730095003_A ID 102412_IFU HS DOXO :print 9/11/12 17:07 Page 4
