Ozone supply air filter, Figure 10-8, M9110eh perma pure – Teledyne 9110EH - Nitrogen Oxides Analyzer User Manual
Page 184: Dryer
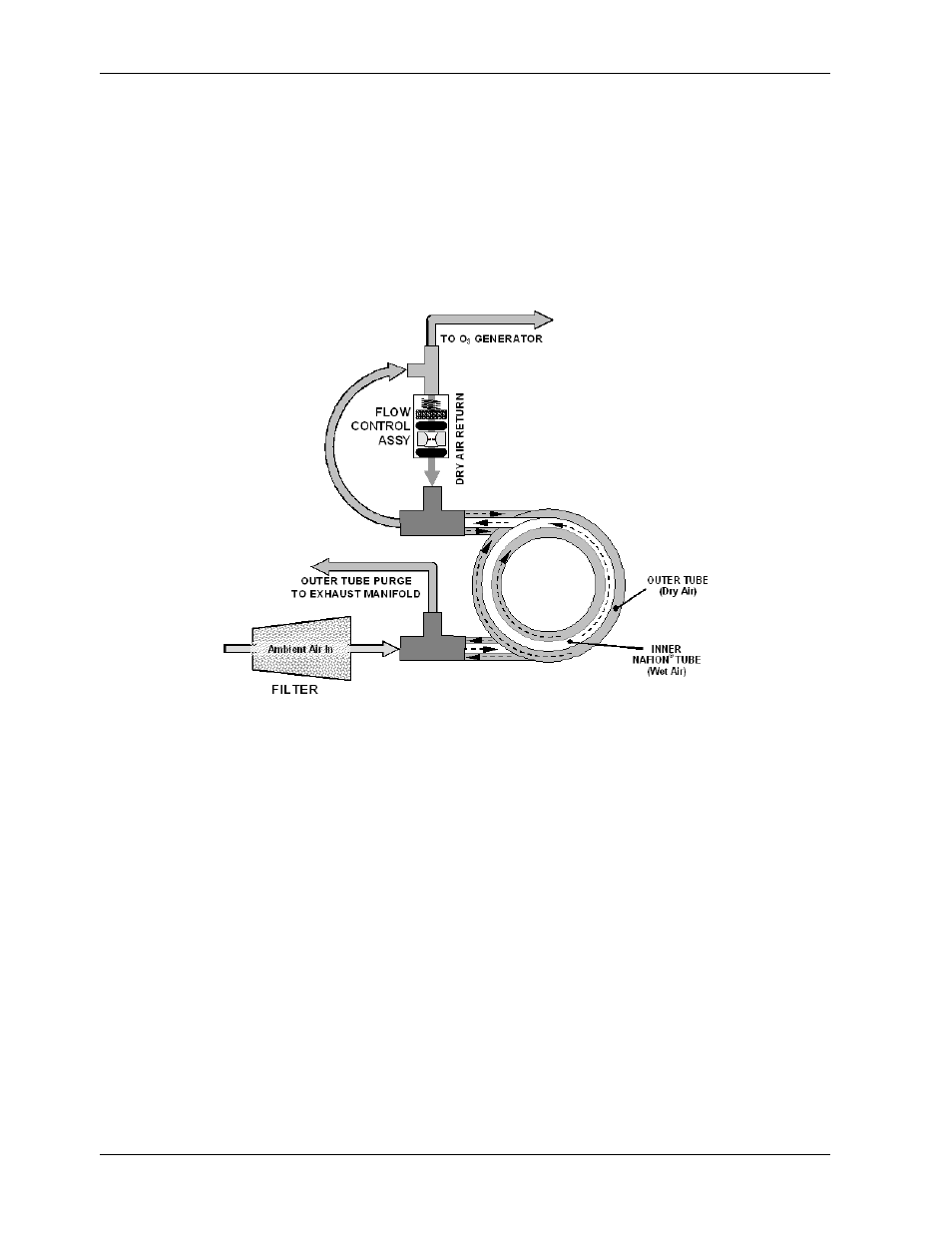
Theory of Operation Model 9110EH Instruction Manual
on hydrogen bonds between the water molecule and the Nafion material. Other small polar
gases that are capable of hydrogen bonds can be absorbed this way, too, such as ammonia
(NH
3
) and some low molecular amines. The gases of interest, NO and NO
2
, do not get
absorbed and pass the dryer unaltered.
To provide a dry purge gas for the outer side of the Nafion tube, the M9110EH returns some of
the dried air from the inner tube to the outer tube (Figure 10-8). When the analyzer is first
started, the humidity gradient between the inner and outer tubes is not very large and the
dryer’s efficiency is low at first but improves as this cycle reduces the moisture in the
sample gas and settles at a minimum humidity.
Figure 10-8: M9110EH Perma Pure
®
Dryer
Just like on startup, if the instrument is turned on after having been off for more than 30
minutes, it takes a certain amount of time for the humidity gradient to become large
enough for the Perma Pure
®
Dryer to adequately dry the air. In this case, called a cold
start, the O
3
Generator is not turned on for 30 minutes. When rebooting the instrument
within less than 30 minutes of power-down, the generator is turned on immediately.
The Perma Pure
®
Dryer used in the M9110EH is capable of adequately drying ambient air to a
dew point of ≤ -5˚C (~4000 ppm residual H
2
O) at a flow rate of 1 standard liter per minute
(slpm) or down to ≤ -15˚C (~1600 ppm residual H
2
O) at 0.5 slpm. The Perma Pure
®
Dryer
is also capable of removing ammonia from the sample gas up to concentrations of
approximately 1 ppm.
10.2.7. Ozone Supply Air Filter
The M9110EH uses ambient air as the supply gas for the O
3
generator and may produce a
variety of byproducts. Small amounts of water, ammonia and various sulfur oxides can
combine to create ammonium sulfate, ammonium nitrate, nitric acid and other compounds.
Whereas sulfates and nitrates can create powdery residues inside the reaction cell causing
sensitivity drift, nitric acid is a very aggressive compound, which can deteriorate the
analyzer’s components. In order to remove these chemical byproducts from the O
3
gas
170 M9110EH Rev 0
