Nox and no2 determination, And no, Determination – Teledyne 9110EH - Nitrogen Oxides Analyzer User Manual
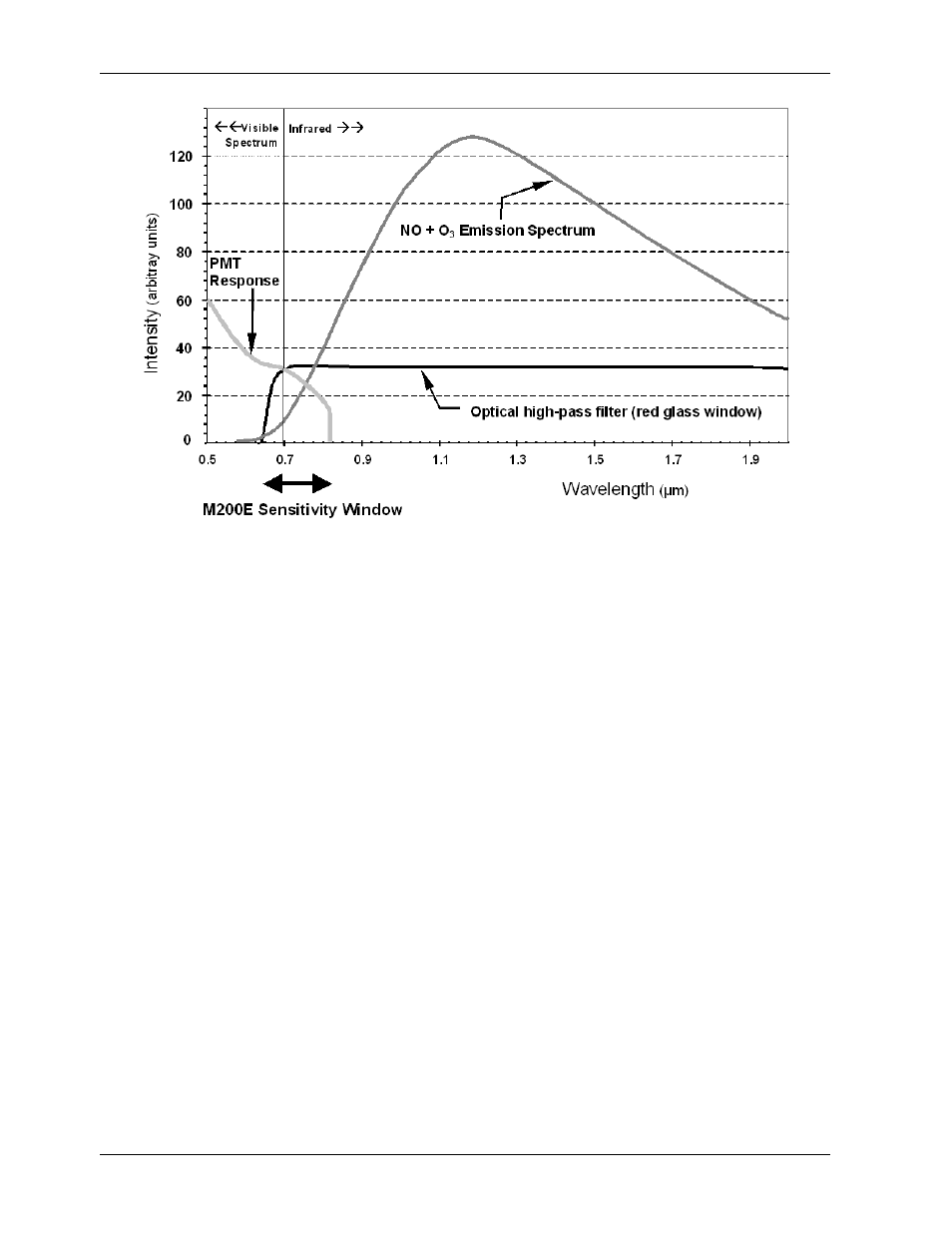
Page 174: Figure 10-1, M9110eh sensitivity spectrum, Mno m no
Theory of Operation Model 9110EH Instruction Manual
Figure 10-1: M9110EH Sensitivity Spectrum
However, only about 20% of the NO
2
that is formed through reaction 10-1 is in the excited
state. In addition, the excited NO
2
can collide with another collision partner M in the
reaction cell (mostly other molecules but also cell walls) and transfer its excess energy to
its collision partner without emitting any light at all (Equation 10-3). In fact, by far the
largest portion of the NO
2
* returns to the ground state this way, leaving only a few percent
yield of usable chemiluminescence.
Eq 10-3)
M
NO
M
NO
+
→
+
2
*
2
In order to enhance the light yield of the reaction, the reaction cell is maintained at reduced
pressure. The probability of a collision between the NO
2
* molecule and a collision partner M
increases proportionally with the reaction cell pressure. This non-radiating collision with the
NO
2
* molecules is usually referred to as quenching, an unwanted process further described
in Section 10.1.5.2.
10.1.2. NO
X
and NO
2
Determination
The only gas that is truly measured in the M9110EH is NO. Any NO
2
contained in the gas is not
detected in the above process since NO
2
does not react with O
3
to undergo chemilumi-
nescence.
In order to measure the concentration of NO or NO
X
(which is defined here as the sum of
NO and NO
2
in the sample gas), the M9110EH periodically switches the sample gas stream
through a converter cartridge filled with molybdenum (Mo, “moly”) chips (Figure 10-6)
heated to a temperature of 315° C. The heated molybdenum reacts with NO
2
in the sample
gas and produces a vriety of molybdenum oxides and NO according to Equation 10-4.
160 M9110EH Rev 0
