Perma pure, Dryer – Teledyne 9110E - Nitrogen Oxides Analyzer User Manual
Page 183
Model 9110E Instruction Manual
Theory of Operation
M9110E Rev B
169
The M9110E utilizes a dual-dielectric design. This method utilizes a glass tube with hollow
walls. The outermost and innermost surfaces are coated with electrically conductive
material. The air flows through the glass tube, between the two conductive coatings, in
effect creating a capacitor with the air and glass acting as the dielectric. The layers of glass
also separate the conductive surfaces from the air stream to prevent reaction with the O
3
.
As the capacitor charges and discharges, electrons are created and accelerated across the
air gap and collide with the O
2
molecules in the air stream splitting them into elemental
oxygen. Some of these oxygen atoms recombine with O
2
to O
3
.
The quantity of ozone produced is dependent on factors such as the voltage and frequency
of the alternating current applied to the CD cells. When enough high-energy electrons are
produced to ionize the O
2
molecules, a light emitting, gaseous plasma is formed, which is
commonly referred to as a corona, hence the name corona discharge generator.
10.2.6. Perma Pure
®
Dryer
The air supplied to the O
3
generation system needs to be as dry as possible. Normal room
air contains a certain amount of water vapor, which greatly diminishes the yield of ozone
produced by the ozone generator. Also, water can react with other chemicals inside the O
3
Generator to produce chemicals that damage the optical filter located in the reaction cell
(Table 10-4) such as ammonium sulfate or highly corrosive nitric acid.
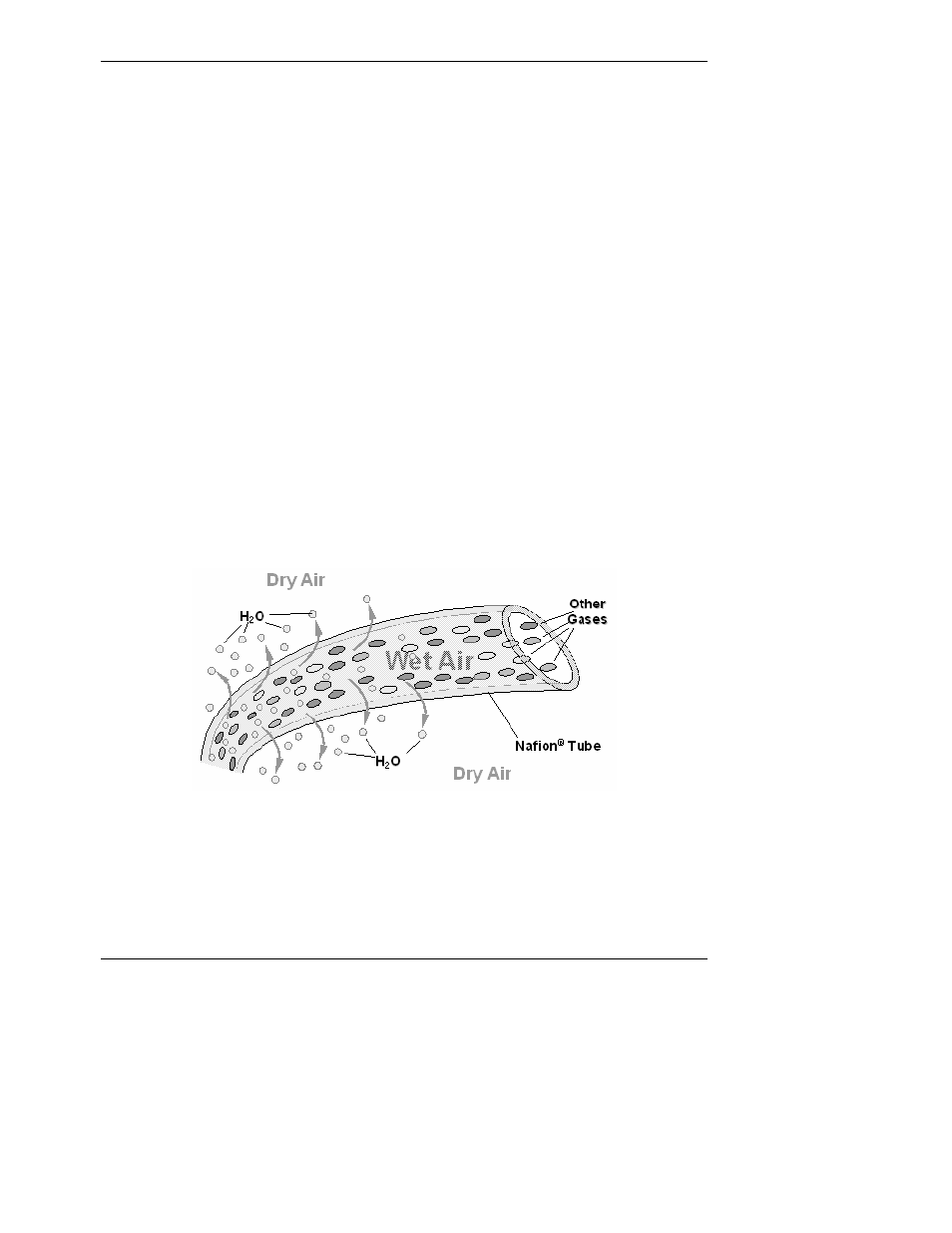
To accomplish this task the M9110E uses a Perma Pure
®
single tube permeation dryer. The
dryer consists of a single tube of Nafion
®
, a co-polymer similar to Teflon
®
that absorbs
water very well but not other chemicals. The Nafion
®
tube is mounted within an outer,
flexible plastic tube. As gas flows through the inner Nafion
®
tube, water vapor is absorbed
into the membrane walls. The absorbed water is transported through the membrane wall
and evaporates into the dry, purge gas flowing through the outer tube, countercurrent to
the gas in the inner tube (Figure 10-7).
Figure 10-7: Semi-Permeable Membrane Drying Process
This process is called per-evaporation and is driven by the humidity gradient between the
inner and outer tubes as well as the flow rates and pressure difference between inner and
outer tubing. Unlike micro-porous membrane permeation, which transfers water through a
relatively slow diffusion process, per-evaporation is a simple kinetic reaction. Therefore, the
drying process occurs quickly, typically within milliseconds. The first step in this process is a
chemical reaction between the molecules of the Nafion
®
material and water, other chemical
components of the gases to be dried are usually unaffected. The chemical reaction is based
