12 conductivity – In-Situ TROLL 9500 Operators Manual User Manual
Page 79
72
Multi-Parameter
Water Quality TROLL
®
0095110 rev. 007 01/09
TROLL 9500 Operator’s Manual
12 CONDUCTIVITY
Ultra-pure distilled water
0.05 μS/cm
Distilled water
1.0 μS/cm
Drinking water
50 to 300 μS/cm
Surface water
100 to 10,000 μS/cm
Sea water
40,000 to 55,000 μS/cm
Great Salt Lake
158,000 μS/cm
Typical Conductivity values
WHAT IS CONDUCTIVITY?
Electrical conductivity measures the ability of a material to carry an
electric current. Lakes, rivers, oceans, and underground aquifers
are typically good conductors because they contain dissolved salts
and minerals. These salts and minerals dissociate in the presence
of water to form negatively and positively charged particles called
anions and cations. Anions and cations provide a pathway for the
transportation of electrical charges throughout the aqueous medium.
For the most part, the higher the concentration of dissolved salts and
minerals in water, the better the conductor and the higher the electri-
cal conductivity. Deionized/distilled water is a poor conductor because
almost all anions and cations are removed during the deionization/dis-
tillation process.
WHY MEASURE CONDUCTIVITY?
Changes in the conductivity of a body of water are often used to indi-
cate an environmental event. For example, a drastic increase in the
electrical conductivity of an underground fresh water aquifer located
near the ocean could indicate the beginning of salt water intrusion.
On the other hand, an increase in the electrical conductivity of a small
lake that is completely surrounded by farmland may simply be the
result of runoff from a recent rain.
HOW IS CONDUCTIVITY MEASURED?
Conductance is the reciprocal of the resistance, in ohms, measured
between two opposing electrodes of a 1 cm cube at a specific temper-
ature. The unit 1/ohm or mho was given the name of Siemens (S) for
conductance. It is not practical to require all conductance cells to have
the dimensions of an exact cube. To enable the comparison of data
from experiments with different conductance cells, the conductance is
multiplied by the cell constant to give conductivity in Siemens per cen-
timeter (S/cm). Cell constants are determined for each sensor using a
standard solution of known conductivity. The cell constant depends on
the electrode area and the amount of separation or distance between
the electrodes.
Early conductivity measurements were performed using cells with two
electrodes. This method required using three conductivity cells with
different cell constants in order to span the range of 1 to 100,000 mi-
croSiemens per centimeter (μS/cm). Another inconvenience occurred
when deposits formed on the electrodes, thus reducing the measured
conductivity of the sample.
The modern four-electrode conductivity cell offers many advantages
over the two-electrode method. It contains two drive electrodes and
two sensing electrodes. The sensing electrodes are positioned in a
low current area so that electrode fouling is minimized. An alternat-
ing current is used to drive the cell. This reduces errors caused by
polarization resulting from the application of a direct current.
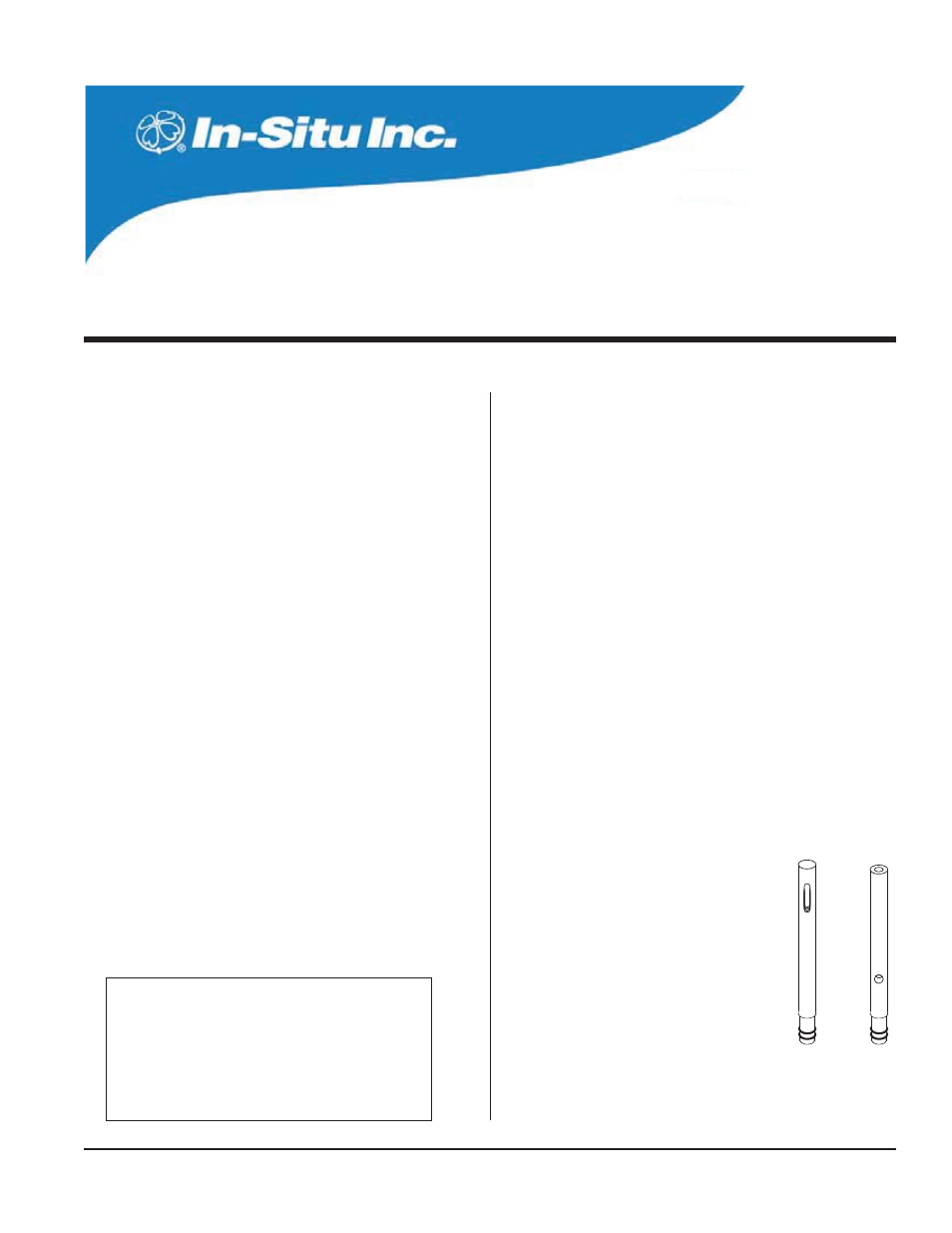
THE CONDUCTIVITY SENSORS
Two conductivity sensors are available, optimized
for performance in different areas of the conductivity
range. Chemically resistant electrodes are used for
lower reactivity in high conducting samples (carbon
electrodes in the low-range sensor, passivated stain-
less steel electrodes in the high-range sensor).
The conductivity sensors are shorter than the
other water quality sensors in order to distance the
conductivity cell from the KCl reference solutions in
other sensors.
Low
High
