Cat o m xno ymo xno, And no, Determination – Teledyne 9110TH - Nitrogen Oxides Analyzer User Manual
Page 278
Model 9110TH NOx Analyzer
Principles
of
Operation
Teledyne Analytical Instruments
258
8.1.2. NO
X
AND NO
2
DETERMINATION
The only gas that is truly measured in the 9110TH/M is NO. Any NO
2
contained in the
gas is not detected in the above process since NO
2
does not react with O
3
to undergo
chemiluminescence.
In order to measure the concentration of NO or NO
X
(which is defined here as the sum
of NO and NO
2
in the sample gas), the 9110TH/M periodically switches the sample gas
stream through a converter cartridge filled with molybdenum (Mo, “moly”) chips heated
to a temperature of 315° C. The heated molybdenum reacts with NO
2
in the sample gas
and produces a variety of molybdenum oxides and NO according to Equation 9-4.
)
315
(
→
2
C
at
O
M
xNO
yMo
xNO
z
oy
(Equation 9-4)
Once the NO
2
in the sample gas has been converted to NO, it is routed to the reaction
cell where it undergoes the chemiluminescence reaction described in Equations 9-1 and
9-2.
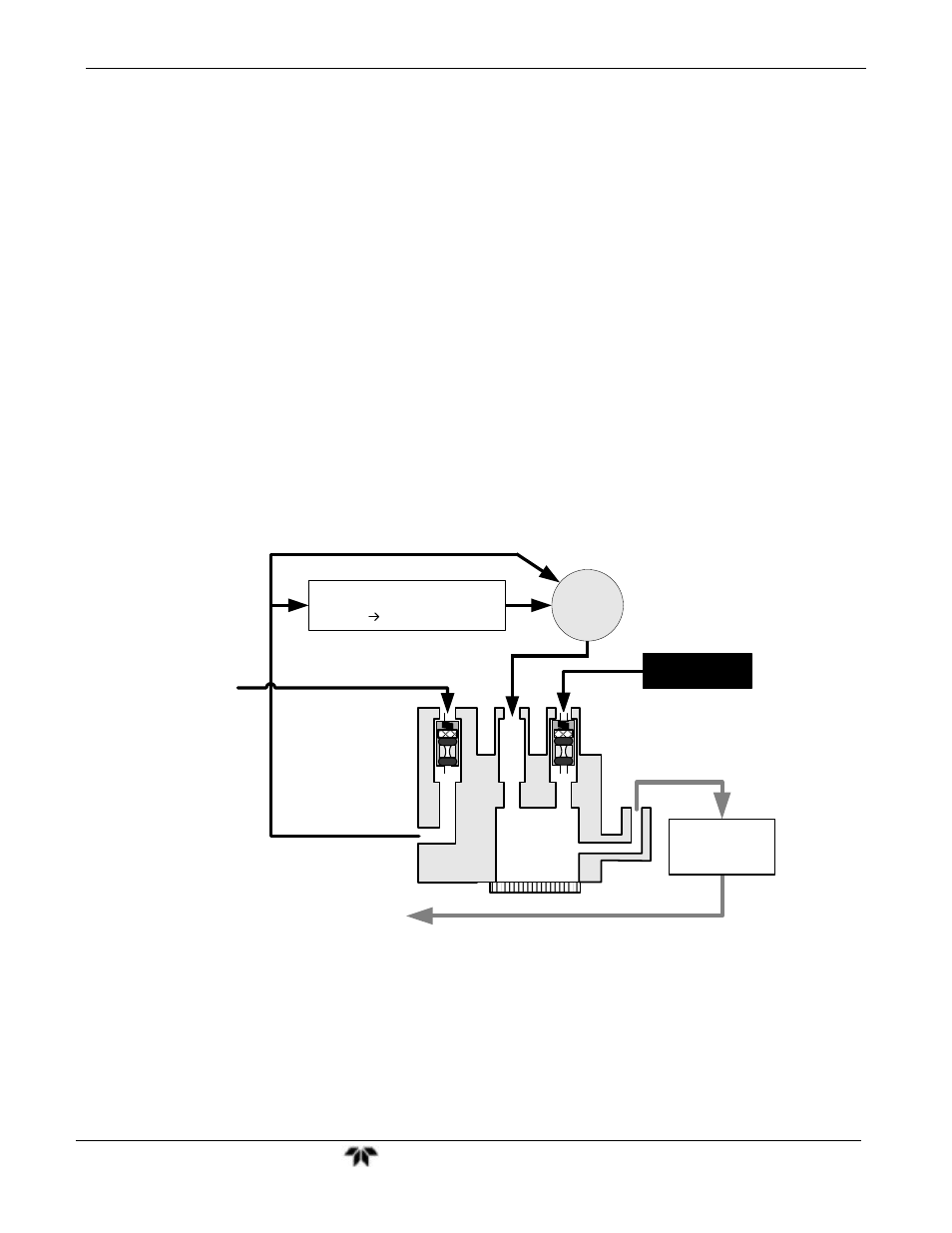
O
3
Generator
REACTION
CELL
O
3
Scrubber
Sample
Gas
(All NO
2
converted to NO)
Exhaust
NO/NO
X
Valve
Molybdenum Converter
NO
2
+ Mo NO + Mo
y
O
2
at 315
o
C
NO + NOx
Figure 8-2:
NO
2
Conversion Principle
By converting the NO
2
in the sample gas into NO, the analyzer can measure the total
NO
X
(NO+NO
2
) content of the sample gas. By switching the NO
2
converter in and out
of the sample gas stream every 6 - 10 seconds, the 9110TH/M analyzer is able to quasi-
continuously measure both the NO and the total NO
X
content.
The NO
2
concentration, finally, is not measured but calculated by simply subtracting the
known NO content of the sample gas from the known NO
X
content.
