9 "ca-mg – Metrohm 794 Basic Titrino User Manual
Page 177
6.3 User methods
794 Basic Titrino
173
6.3.9 "Ca-Mg"
Determination of the hardness of drinking wa-
ter
Electrode:
6.0504.100 Ca electrode and 6.0726.100
Ag/AgCl reference electrode (outer electrolyte
KNO
3
sat.), at measuring input 1.
Titrant:
c(Na
2
EDTA) = 0.1 mol/L in c(KOH) = 0.1
mol/L
Aux. reagent:
c(acetyl acetone) = 0.1 mol/L + c(TRIS) =
0.2 mol/L (TRIS = trishydroxymethyl amino-
methane)
Sample:
100 mL drinking water,
add 15 mL auxiliary reagent.
Remarks:
The volume of the auxiliary reagent can be
optimized: As a rule of thumb, the ratio
Mg/acetyl acetone should be app. 0.05.
Reference:
Metrohm Application Bulletin Nr. 125
– Calcium hardness in mmol/L
– Magnesium hardness in mmol/L
– Total hardness in mmol/L
– Sample size in mL
– Concentration of the titrant
– Factor for mmol
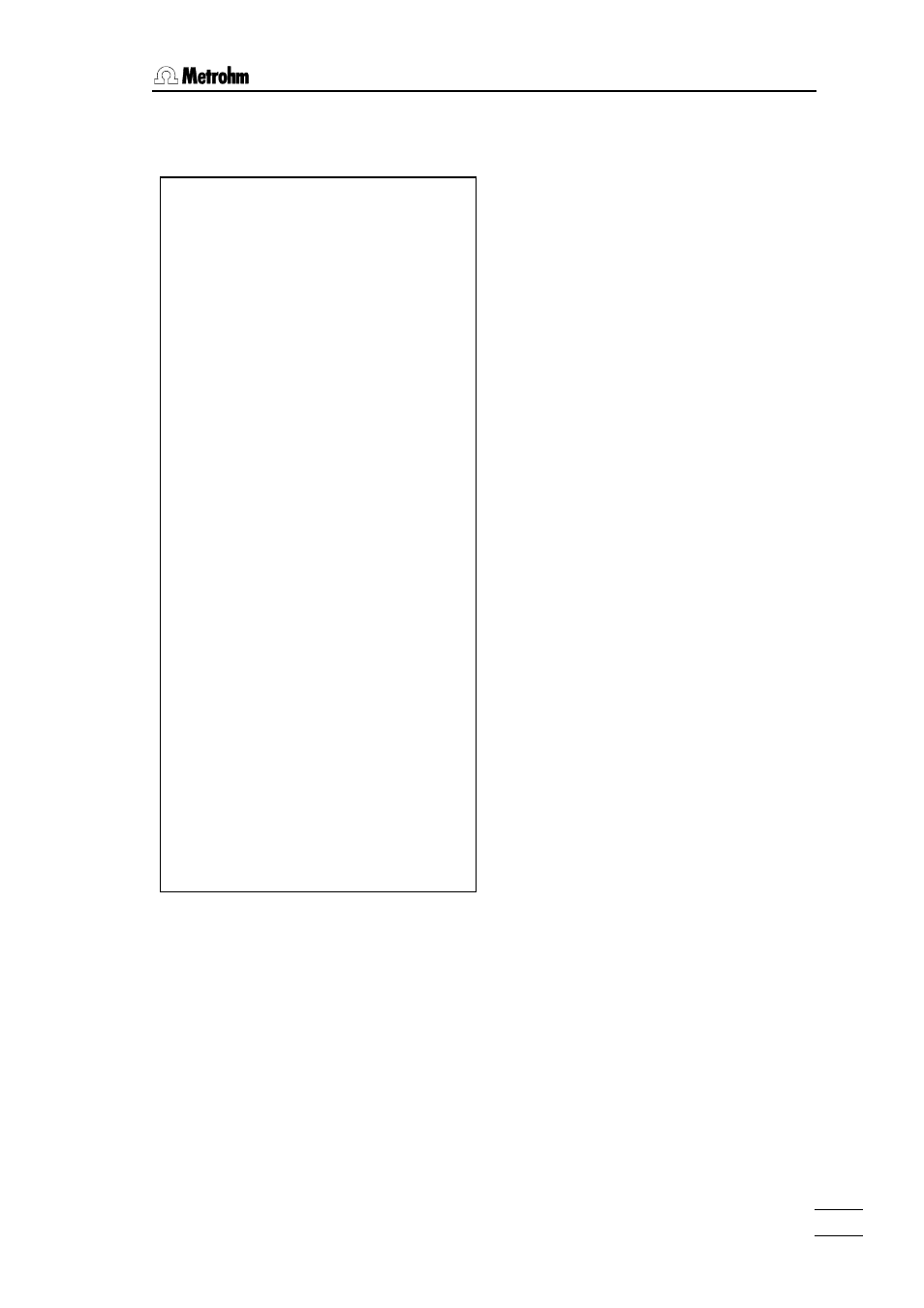
'pa
794 Titrino 01102 794.0010
date 2002-01-03 time 09:52 0
DET U Ca-Mg
parameters
>titration parameters
meas.pt.density 1
min.incr. 10.0 µl
titr.rate max. ml/min
signal drift 20 mV/min
equilibr.time 38 s
start V: OFF
pause 0 s
meas.input: 1
temperature 25.0 °C
>stop conditions
stop V: abs.
stop V 5 ml
stop U OFF mV
stop EP 9
filling rate max. ml/min
>statistics
status: OFF
>evaluation
EPC 5
EP recognition: all
fix EP1 at U OFF mV
pK/HNP: OFF
>preselections
req.ident: OFF
req.smpl size: all
activate pulse: OFF
------------
'fm
794 Titrino 01102 794.0010
date 2002-01-03 time 09:52 0
DET U Ca-Mg
>calculations
Ca++=EP1*C01*C02/C00;2;mmol/l
Mg++=(EP2-EP1)*C01*C02/C00;2;mmol/l
Total=EP2*C01*C02/C00;2;mmol/l
C00= 1.0
C01= 0.05
C02= 1000
------------
