3 solvent data, 3 solvent, Data – Heidolph LABOROTA 4000 up to 4003 User Manual
Page 138: Appendix, J/g] vacuum for boil.at 40 °c
Appendix
136
Laborota 4000/4001 efficient, 4010/4011 digital, 4002/4003 control
1.02
11.3 Solvent
data
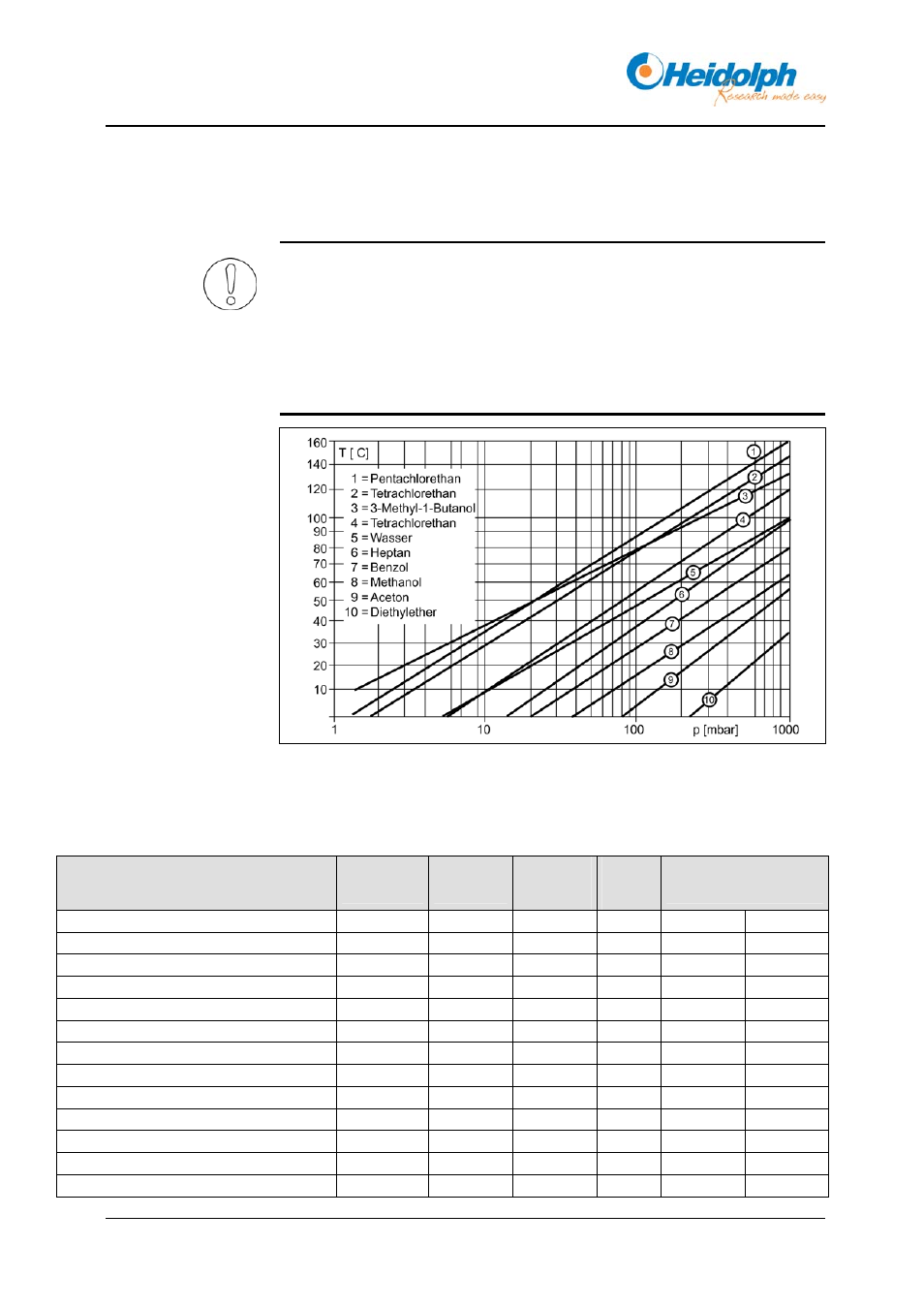
The nomogram shows the relationship between pressure and boiling
temperature of a selection of solvents.
The difference between the temperature of the vapour and the cooling medium
should be 20 K to ensure adequate condensation.
The temperature difference between the heating bath and the vapour should
be at least 20 K to ensure an adequate distillation rate.
As a rule of thumb, doubling the temperature difference will double the
distillation rate.
Fig. 11-1: Nomogram
Conversion Torr to mbar: [mmHg] ≈ 3/4 [mbar]
Solvent data
Solvent
Empirical
formula
MW
[g/mol]
Boil.
[°C]
ΔH
vap
[J/g]
Vacuum for boil.at
40 °C
[mbar]
[mm(Hg)]
Acetone C
3
H
6
O 58.08
56.5
550
556 387
Acetonitrile C
2
H
3
N 41.05
81.8
833
230 173
Benzene C
6
H
6
78.11
80.1
549
236 177
n-butanol (butyl alcohol)
C
4
H
10
O
74.12
117.5
619
25 19
Tert. butanol (tert. butyl alcohol)
C
4
H
10
O
74.12
82.9
588
130 98
2-butanone (methyl ethyl ketone)
C
4
H
8
O 72.11
79.6
473
243 182
Chlorobenzene C
6
H
5
CI 112.60
132.2
375
36
27
Cyclohexane C
6
H
12
84.16
80.7
389
235 176
1,2-dichloroethane C
2
H
4
CI
2
98.96
82.4
336
210 158
1,2-dichloroethylene (cis)
C
2
H
2
CI
2
96.94
59.0
320
479 134
1,2-dichloroethylene (trans)
C
2
H
2
CI
2
96.94
47.8
313
751 563
Dichloromethane (methylene dichloride)
CH
2
CI
2
84.93
40.7
373
atm. atm.
