Section 8 principles of operation, 1 chemistry – YSI 9600 User Manual
Page 70
SECTION 8 PRINCIPLES OF OPERATION
8.1 CHEMISTRY
The YSI 9600 uses a standard colorimetric method to measure combined nitrate and nitrite levels in natural
water samples. Nitrate is converted to nitrite by cadmium reduction and the nitrite subsequently reacts with
sulfanilamide and N-naphthyl ethylenediamine to form a colored species with a 540 nm absorption
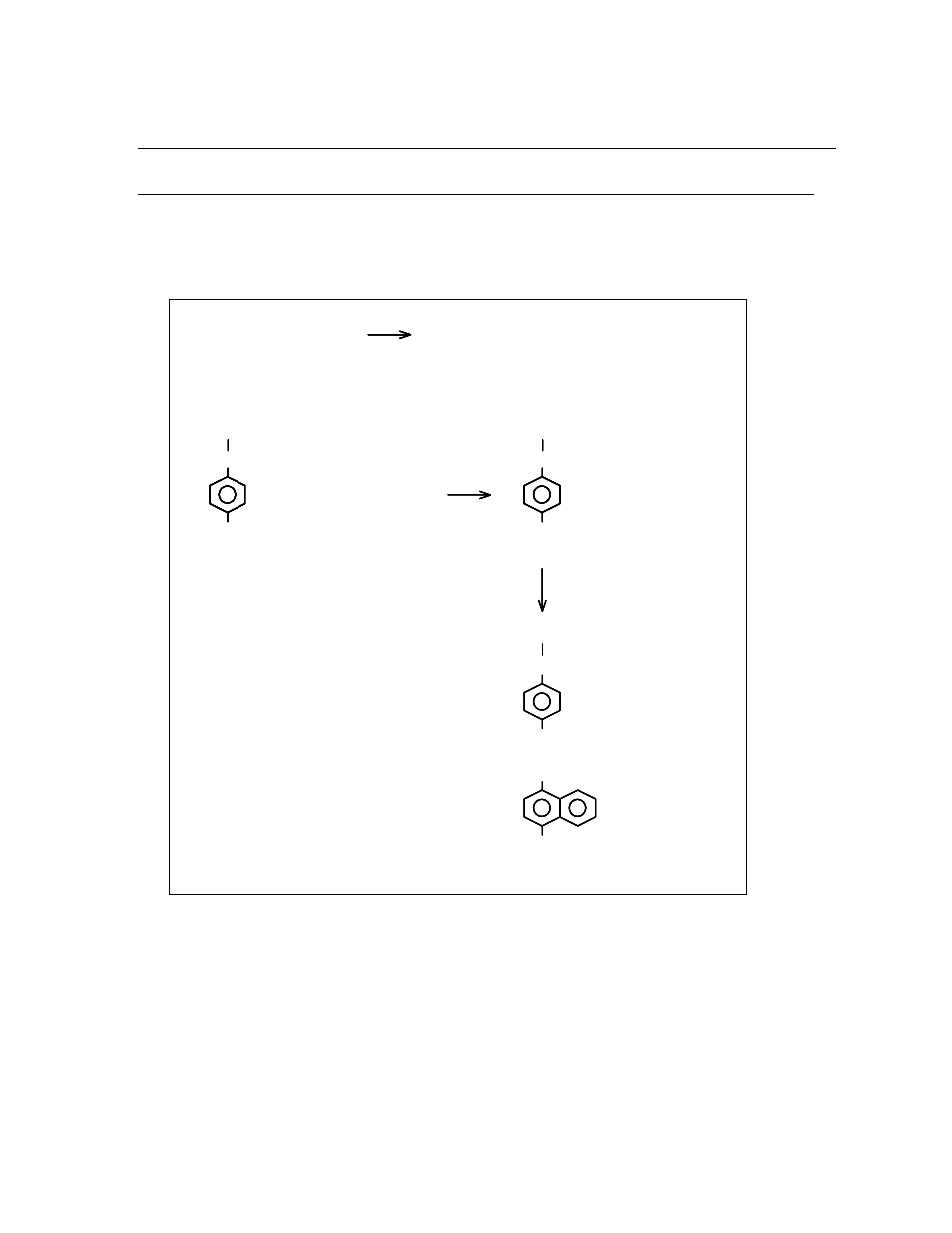
maximum as shown in the diagram below:
-
2
NO + 2H + 2e NO + H O
-
3
+
-
Cd
2
2
O = S = O
NH
2
N N Cl
+
-
-
2
O = S = O
NH
2
+ NO + 2 HCl + 2 H O + Cl
-
2
NH
2
NED
O = S = O
NH
+ HCl
N
N
=
HNCH CH NH
2
2
2
–
–
–
-
2
NO + 2H + 2e NO + H O
-
3
+
-
Cd
2
2
O = S = O
NH
2
N N Cl
+
-
-
2
O = S = O
NH
2
+ NO + 2 HCl + 2 H O + Cl
-
2
NH
2
NED
O = S = O
NH
+ HCl
N
N
=
HNCH CH NH
2
2
2
-
2
NO + 2H + 2e NO + H O
-
3
+
-
Cd
2
-
2
NO + 2H + 2e NO + H O
-
3
+
-
Cd
2
NO + 2H + 2e NO + H O
-
3
+
-
Cd
NO + 2H + 2e NO + H O
-
3
+
-
NO + 2H + 2e NO + H O
-
3
NO + 2H + 2e NO + H O
-
3
+
-
Cd
2
2
O = S = O
NH
2
N N Cl
+
-
-
2
O = S = O
NH
2
+ NO + 2 HCl + 2 H O + Cl
-
2
NH
2
NED
O = S = O
NH
+ HCl
N
N
=
HNCH CH NH
2
2
2
2
O = S = O
NH
2
O = S = O
NH
O = S = O
NH
O = S = O
O = S = O
NH
2
N N Cl
+
-
N N Cl
N N Cl
N N Cl
+
-
-
2
O = S = O
NH
2
+ NO + 2 HCl + 2 H O + Cl
-
2
NH
2
O = S = O
NH
2
O = S = O
NH
O = S = O
NH
O = S = O
O = S = O
NH
2
+ NO + 2 HCl + 2 H O + Cl
-
2
+ NO + 2 HCl + 2 H O + Cl
-
2
NH
2
NH
2
NED
O = S = O
NH
+ HCl
N
N
=
HNCH CH NH
2
2
2
+ HCl
N
N
=N
N
=
HNCH CH NH
2
2
2
–
–
–
Efficient reduction of the nitrate ion by cadmium requires the use of “copper activated” cadmium.
Cadmium metal is treated with a Cu
+2
solution resulting in deposition of elemental copper on the cadmium.
This results in what is believed to be a copper/cadmium galvanic cell with reduction occurring at copper
(1).
(1) Nydahl, F. Talanta 1976, 23, 349-357.
YSI Environmental
70
Model 9600 Nitrate Monitor
