Complex formation – Metrohm 757 VA Computrace User Manual
Page 186
8 Troubleshooting
757 VA Computrace – Software
176
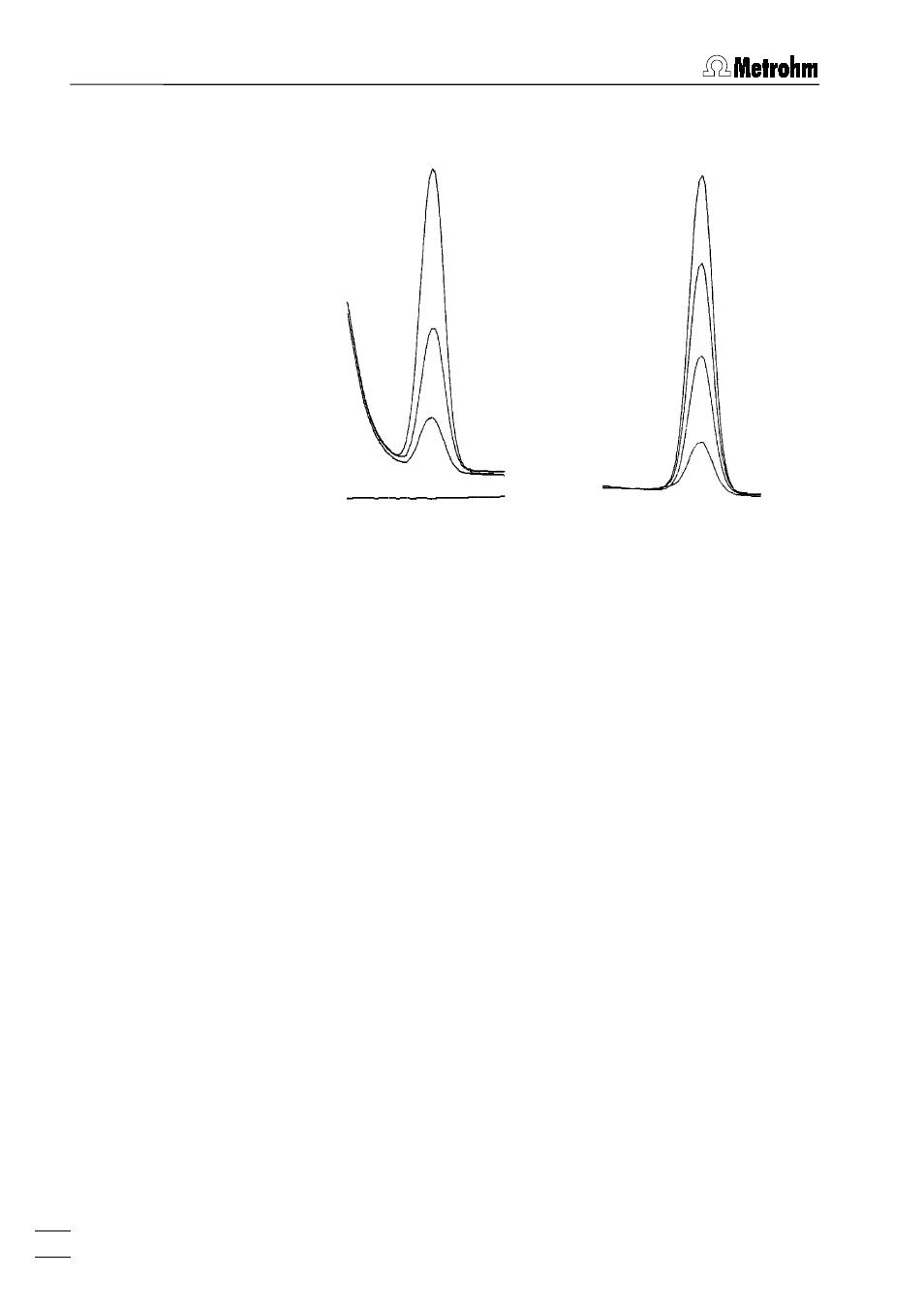
Supporting electrolyte:
Supporting electrolyte:
0.01 mol/L HClO4; pH = 2
acetate buffer pH = 4.64
Analysis solution:
deionised water
Standard addition:
with 50 ng Zn
Electrode:
HMDE (enrichment 60 s at –1.2 V)
Complex formation
Substances determined polarographically can occur in various
complexed forms, depending on the composition of the analysis
solution. As complexing is always associated with a shift in the
half-wave potential and the limiting current, difficulties can arise in
the peak evaluation. Such difficulties must be eliminated by ap-
propriate changes in the composition of the supporting electro-
lyte.
If it is not possible to remove the interfering complexing agents
from the analysis solutions or to mask them by suitable sub-
stances, it is often helpful to change the pH of the supporting
electrolyte. Another measure which is often used involves the ad-
dition of a ligand of high complexing power (e.g. EDTA) to bring
about 100% change of the analyte to a definitive form. The latter
possibility is also used in the following example:
• Copper determination in chloride-containing solutions
Copper can occur in chloride-containing solutions as both a
CuCl
4
2–
and a CuCl
2
–
complex. The two associated current
peaks are near each other. In unfavourable cases, the deter-
mination of copper is not possible. The difficulties disappear
after addition of the complexing agent EDTA as now all cop-
per is completely in the form of a Cu-EDTA complex. (Increas-
ing the chloride concentration [e.g. by addition of 1 mL of a
1.5 mol/L KCl solution of the greatest possible purity per 10
Zn
Zn
