Merit Medical Embosphere Microspheres Sterile Vial IFU-US User Manual
Page 7
7
Quality of Life
The SF-12 Health Status questionnaire was used to assess
changes in general physical and mental health status following
treatment. The goal of this endpoint was to demonstrate at least
a moderate improvement in the overall quality of life by the 6-
month evaluation.
Pre-treatment average scores for the UFE
group were 44.4 ± 8.5 and 45.1 ± 11.9 for the physical and
mental components, respectively, putting this group at slightly
better than the 25th percentile (44.32) of normalized scores
published for the U.S. population of females in general. By 6
months, the average scores for both the physical and mental
questionnaires had increased significantly to 52.2 ± 6.7 and
52.4 ± 8.8, respectively, putting the UFE group at the 50th
percentile (52.76) of normalized SF-12 scores (p<0.001 for both
measures).
Secondary Efficacy Endpoints
Fibroid and Uterine Volume
UFE patients underwent uterine imaging by MRI or ultrasound
at baseline and follow-up. Uterine and fibroid volumes were
calculated using the formula for the volume of a prolate ellipse
(LxWxDx0.52). Significant decreases in both uterine volume
(measured as including the cervix) and uterine fibroid volume
were recorded for the UFE group by the 3-month evaluation,
with further improvements seen at 6 months (p<0.001 at both
time periods as compared to baseline). Table 4 summarizes the
percent changes in uterine and fibroid volumes at 6 months
following treatment. This table includes uterine volume data
from 91 of the 108 UFE Phase II patients (84%) and fibroid
volume data from 83 of these patients (77%) who had complete
and evaluable imaging reports at baseline, and at 3 months and
6 months following UFE treatment.Increases in uterine volumes
were reported for 11 patients (12%) and increases in fibroid
volumes for 8 patients (8%) by the 6-month evaluation.
Table 4 - Percent Change in Uterine and Fibroid Volumes From
Baseline
A positive percent change indicates a decrease in volume, while
a negative percent change indicates an increase in volume.
Patient Satisfaction
Both study groups showed a high level of satisfaction with the
outcome of their procedures at both 3 and 6 months. Ninety-
two of 100 UFE patients (92%) and 46 of 47 hysterectomy
patients (98%)
who completed the patient satisfaction
questionnaire at 6 months were slightly, moderately or very
satisfied with the outcome of their procedure, with the majority
in both groups being very satisfied.
ADVERSE EVENTS:
Adverse event data is reported for all 132 patients treated by
UFE in both Phases I and II.
There were no unanticipated
adverse device effects or unanticipated adverse events reported
in this study. Table 5 presents fifty-one adverse events judged
to be probably or possibly associated with the procedure, which
occurred in 37 of the 132 UFE patients (28%). Seven of the 51
events (14%) occurred during the UFE procedure, five (10%)
between the procedure and hospital discharge, 17 (33%) from
hospital discharge to 1-month post-procedure, 11 (22%) from
1 to 3 months post-procedure, 4 (8%) from 3 to 6 months post-
procedure, and 7 (14%) greater than 6 months post-procedure.
The most common adverse event was an allergic reaction or
rash, which occurred in 8 of the 132 patients (6%), and which
were generally judged by the treating physician to be related to
the drugs or contrast agent used during the procedure.
All
reactions resolved spontaneously or with treatment.
Four
patients had hysterectomies following UFE, for an overall
hysterectomy rate of 3%. One hysterectomy was performed at
2 months post-UFE due to sustained fever/possible infection.
The other three were elective hysterectomies due to
dissatisfaction with UFE outcome, which occurred at 2, 10, and
11 months post-UFE. One patient (<1%) had a repeat UFE after
her uterine arteries were found to be patent.
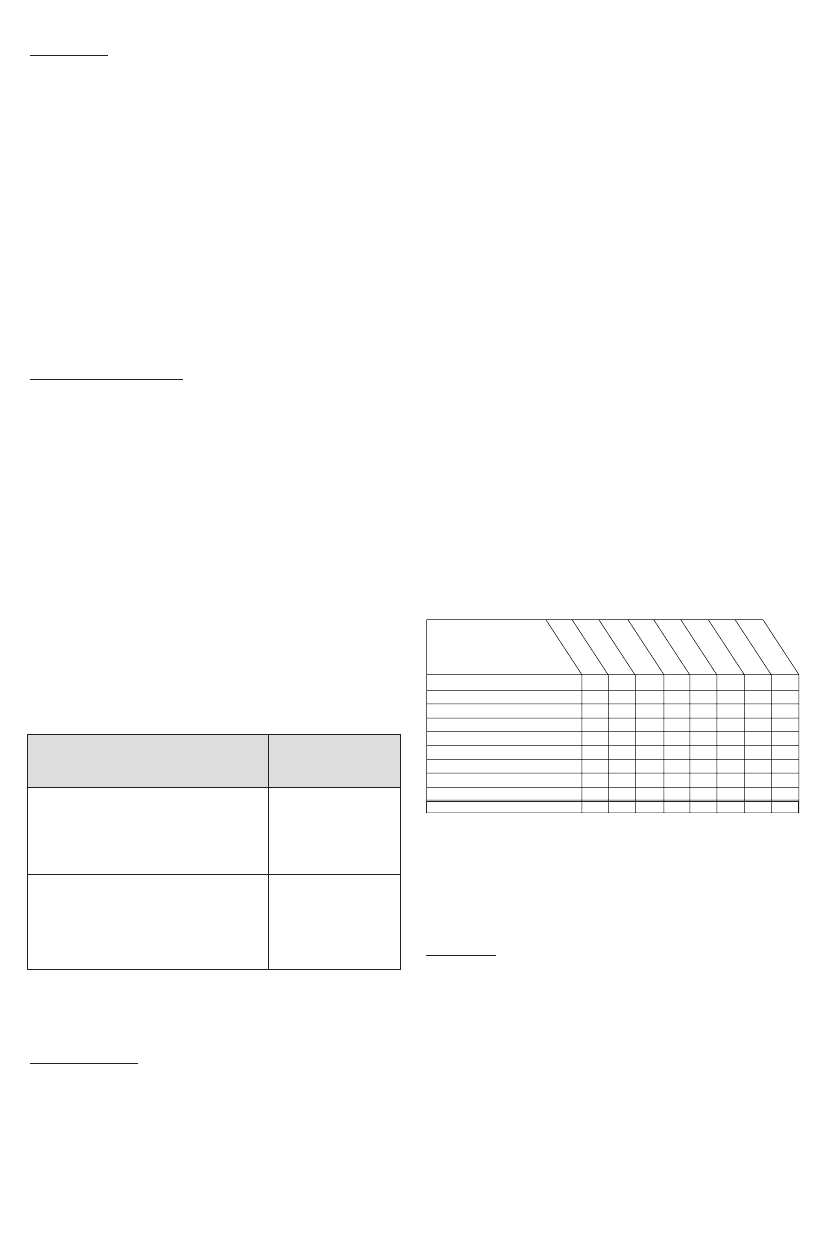
Table 5 - Timing and Type of Probably or Possibly UFE-Related
Adverse events
total of 34 out of 132 patients (26%) experienced one or more
adverse event in this study.
The number of patients in this
column reflects the fact that some patients experienced more
than one adverse event.
REFERENCES:
UFE Specific
1. Spies J et al., Initial experience with use of tris-acryl gelatin
microspheres for uterine artery embolization for leiomyomata, J
Vasc Interv Radiol, 12:1059-1063, 2001.
2. Spies J et al., Complications after uterine artery embolization for
leiomyomas. Obstet Gynecol, 100:873-80, 2002.
3. Goldberg J, Pereira L, and Berghella V: Pregnancy After Uterine
Artery Embolization. Obstet Gynecol, 100(5):869-872, 2002.
4. Scialli A: Alternatives to hysterectomy for benign conditions.
Int J Fert & Women's Med, 43(4):186-91, 1998.
5. Nikolic B, Spies JB, Campbell L, et al.:
Uterine artery
embolization: reduced radiation with refined technique. J Vasc
% Decrease at
6 Months
Uterine Volume (cc)
N
mean (SD)
range
91
33.2% (30.5%)
-93.6% to 82.0%
Fibroid Volume
N
mean (SD)
range
83
50.9% (41.7%)
-173.4% to 99.7%
Event Description
Time to Event
#
Pa
tie
nt
Co
m
pla
in
ts
*
#
Ev
en
ts
Pr
oc
ed
ure
In
H
os
pit
al
<
1
M
on
th
1-
3
M
on
th
s
3-
6
M
on
th
s
>
6
M
on
th
s
Hysterectomy following UFE
4
4
8
8
6
6
7
7
7
7
17
11
5
51
5
5
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
9
9
3
3
3
3
3
4
4
4
4
4
4
Allergic reaction/Rash
Fibroid/Tissue passage or removal
Pain related adverse events
Catheter/puncture site related injury
Urinary tract infection/Cystitis
Vaginal infection/Vaginitis
Vaginal Irritation/Burning/Discharge
Other
Total
