Merit Medical Embosphere Microspheres Sterile Vial IFU-US User Manual
Page 5
5
UFE CLINICAL STUDY SUMMARY:
Study Design
A prospective multi-center trial was conducted to study UFE using
Embosphere Microspheres for treatment of symptomatic uterine
fibroids. A total of 132 women who desired to keep their uterus and
avoid surgery were treated by UFE in the study: 30 in an initial
feasibility study and 102 in the pivotal study. A concurrent, non-
randomized group of 50 patients undergoing hysterectomy was
also included for comparison of safety to the UFE group. Eleven
investigational sites participated in the study: seven of which
performed UFEs and six of which performed hysterectomies.
The study was designed to determine whether UFE using
Embosphere Microspheres could reduce symptoms associated
with the symptomatic fibroids, such as abnormal bleeding, pain,
discomfort, and urinary problems.
Primary study endpoints included:
• Reduction in menstrual bleeding from baseline to 6 months post-
UFE as measured using a Pictorial Bleeding Assessment Chart
(PBLAC)
• Improvement
in
bulk
symptoms
(pelvic
pain,
pelvic
discomfort/bloating, and urinary dysfunction) as measured using a
patient symptom questionnaire
• Improvement in quality of life as measured using the SF-12 Health
Status Questionnaire
Secondary endpoints included:
• Other measures of changes in menstrual bleeding
• Reduction of uterus and fibroid size
• Hospitalization time
• Time to return to normal activities
• Evaluations of patient satisfaction with the procedure
Adverse events and complications were also evaluated with respect
to type, rate, and severity.
Eligibility criteria included age between 30 and 50 years, inclusive,
infertile or no plans to become pregnant, one or more symptomatic
uterine fibroids, uterine volume
≥ 250 cc or fibroid volume ≥ 4 cc,
and baseline PBLAC
≥ 150. Women were excluded from the study
if they were pregnant, had a history of pelvic inflammatory disease,
submucosal fibroid(s) with more than 50% growth into the uterine
cavity, pedunculated serosal fibroid(s) as the dominant fibroid(s),
significant collateral feeding by vessels other than uterine artery,
adenomyosis as the dominant cause of symptoms, endometrial or
pre-malignant hyperplasia, any malignancy of the pelvic region, any
active infection of the pelvic region, known allergy to IV contrast or
gelatin,
bleeding
diathesis,
immunocompromised,
post-
menopausal or baseline FSH > 40 mIU/mL, or treatment with GnRH
agonist within the previous 3 months.
Pre-treatment evaluations included routine gynecological exam and
testing, standard laboratory testing, ultrasound or MRI, menstrual
bleeding record (UFE group), and self-assessment questionnaires
relating to overall health (SF-12), menstrual bleeding, and fibroid
symptoms. Patients were evaluated at 1-3 weeks, 3 months, 6
months and 12 months. PBLAC scores were obtained at 3 and 6
months.
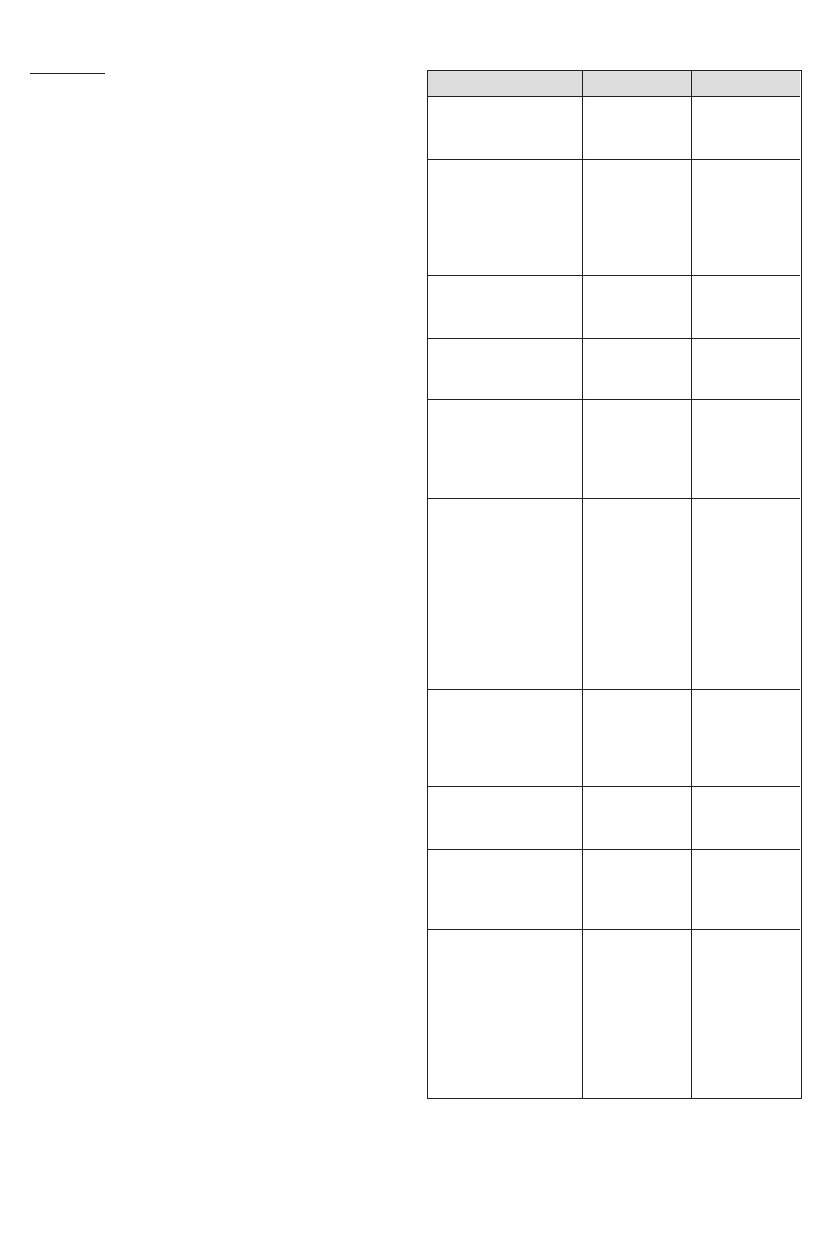
Table 1 - Patient Demographics
*Eighty-four percent of the UFE patients and 98% of the hysterectomy
patients had baseline uterine volumes of 1000 cc or less.
AGE IN YEARS
Mean (SD)
Range
ETHNIC ORIGIN
Asian/Pacific Island
African American
Hispanic
Caucasian
Other
HEIGHT (cm)
Mean (S.D.)
Range
WEIGHT (kg)
Mean (S.D.)
Range
MENSTRUAL STATUS
Frequent
Infrequent
Regular
Unknown
PRIOR FIBROID
TREATMENT
None
GnRH agonist
Oral contraceptive
Other hormonal
Myomectomy
D & C
Hysteroscopy
Other invasive
NUMBER OF FIBROIDS
1
2
≥ 3
no response
UTERINE VOLUME (cc)*
Mean (SD)
Range
DOMINANT FIBROID
VOLUME (cc)
Mean (SD)
Range
FIBROID TYPE
Intramural
Subserosal
Submucosal
Transmural
Pedunculated
More than one type
indicated for some
patients
UFE
42.4 (4.2)
30-50
1 (1%)
67 (59%)
7 (6%)
35 (31%)
3 (3%)
159.9 (10.5)
131-186
72.7 (16.2)
46-123
8 (8%)
1 (1%)
93 (91%)
0 (0%)
53 (52%)
9 (9%)
25 (25%)
9 (9%)
20 (20%)
17 (17%)
13 (13%)
9 (9%)
28 (25%)
37 (33%)
48 (42%)
0 (0%)
692.4 (462.8)
185.6-3076.3
147.4 (154.3)
5.1-776.8
69 (61%)
20 (18%)
18 (16%)
11 (10%)
2 (2%)
Hysterectomy
41.6 (5.3)
31-50
2 (4%)
9 (18%)
8 (16%)
31 (62%)
0 (0%)
161.8 (10.1)
132-178
75.1 (21.5)
50-146
14 (28%)
2 (4%)
33 (66%)
1 (2%)
35 (70%)
2 (4%)
5 (10%)
5 (10%)
4 (8%)
1 (2%)
2 (4%)
3 (6%)
20 (40%)
19 (38%)
10 (20%)
1 (2%)
389.2 (521.2)
91.8-3415.1
90.6 (354.8)
3.2-2322.3
32 (64%)
8 (16%)
13 (26%)
1 (2%)
4 (8%)
