Merit Medical Embosphere Microspheres Sterile Vial IFU-US User Manual
Page 6
6
Study Results
The study results are presented below for 107 patients
considered to be in the Phase II UFE study cohort, which
consisted of 11 UFE Phase I patients who met the Phase II
eligibility criteria and 96 evaluable UFE Phase II patients.
Procedure, Discharge, and Recovery Information
All UFE procedures were technically successful with no
intraoperative complications that prevented completion of the
procedure. The majority (77%) of the UFE procedures were
performed using a 5 Fr catheter with either a 4 Fr (19%) or 3 Fr
(3%) in the remainder. Seventy-two patients were treated with
500-700 micron spheres, 66 patients with 700-900 micron
spheres and 18 patients with 900-1200 micron spheres. Many
of the patients were treated with more than one sphere size.
The most common treatment approach was to start with a
smaller sphere size and then to increase the size if necessary.
The volume of spheres required varied inversely with the sphere
size as an average of 7.2 cc of 500-700 micron spheres was
used as compared to 6 cc of 700-900 micron spheres and 4.1
cc of 900-1200 micron spheres.
The majority of UFE patients underwent the procedure while
under conscious sedation with a local anesthetic given at the
puncture site.
No UFE procedures were performed under
general anesthesia. The average UFE procedure time from first
arterial puncture to final catheter removal was 58 ± 28 minutes
(range 10-140 minutes).
By comparison, all of the
hysterectomy surgeries were performed under general
anesthesia, regardless of the type of hysterectomy performed,
and the average surgery time from skin incision to skin closure
was 93 ± 38 minutes (range 35-171 minutes) (p<0.001). The
majority
of
the
hysterectomy
procedures
were
done
abdominally (76%).
Eighty-seven percent of the UFE patients were discharged from
the hospital on the day following the embolization procedure
and 12% on the same day as the procedure. Hysterectomy
patients spent a significantly longer time in the hospital
(p<0.001), with an average stay of 2.3 days as compared to 0.9
days for the UFE patients. UFE patients were back to work in an
average of 10.7 days, however, this took an average of 30.7
days for the hysterectomy patients (p<0.001). Similarly, the
UFE patients returned to normal daily activities more than three
times quicker than the hysterectomy patients (mean 10.9 days
for UFE versus 37.4 days for hysterectomy, p<0.001).
Primary Efficacy Endpoints
Menstrual Bleeding
To be eligible for UFE in this study, patients were required to
have abnormally heavy menstrual bleeding, with a baseline
score of
≥ 150 on the Pictorial Bleeding Assessment Chart
(PBLAC) of Janssen et al. (1995).
Success was defined as
≥ 50% reduction in PBLAC score by the 6-month follow-up
evaluation.
Additional measures were also used to assess
changes in menstrual bleeding, including patient self-
assessment of their bleeding level and a menorrhagia
questionnaire.
Changes in menstrual bleeding generally occurred quickly
following UFE, with 92% of the patients showing some
improvement by 3 months.
Table 2 presents the menstrual
bleeding success rates at 6 months in the intent-to-treat
population. The data in Table 2 reflects completed PBLACs from
90 of the 107 Phase II UFE patients (84%). Six patients (6%)
did not complete a 6-month PBLAC because they were either
lost to follow-up (n=4) or had a hysterectomy (n=2) prior to this
evaluation period. The eleven remaining patients (10%) without
6-month PBLAC scores had sufficient information from the
other menstrual bleeding assessments to determine their level
of success on this endpoint.
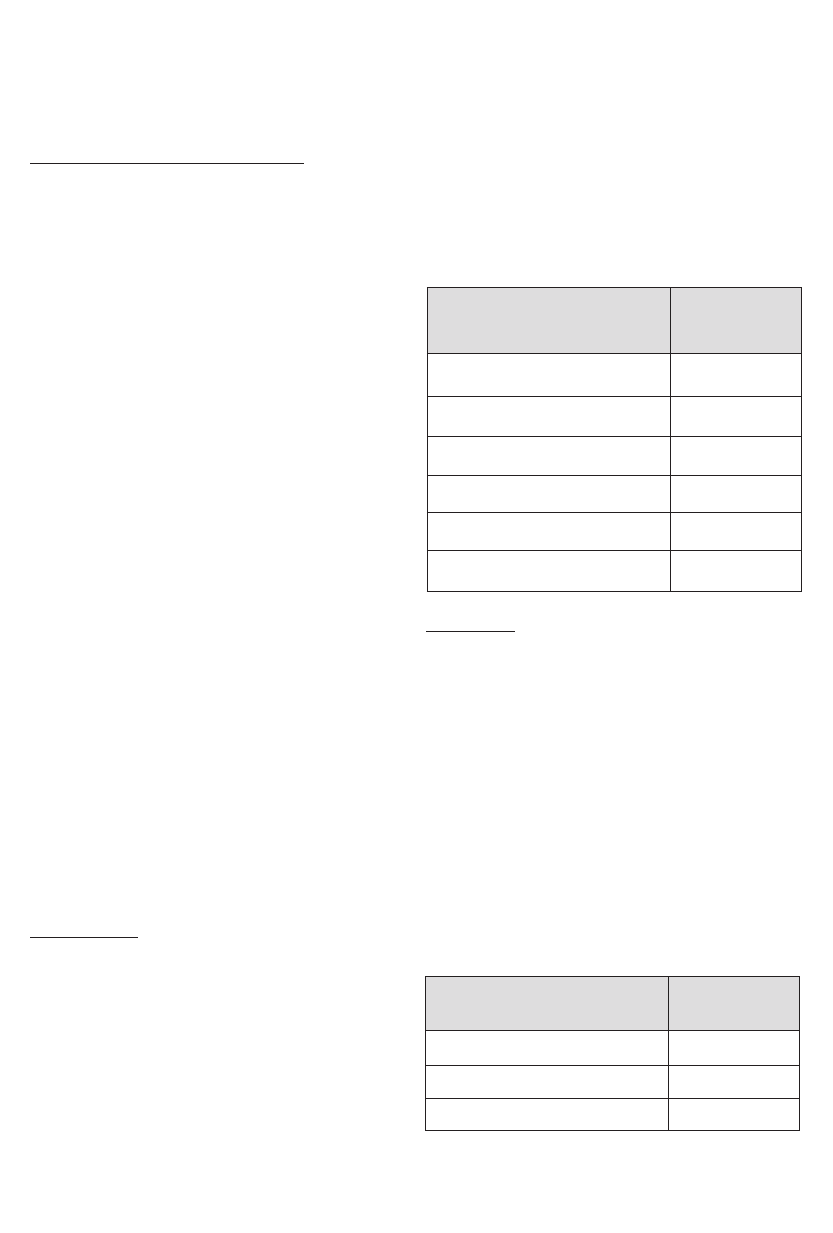
Table 2 - Efficacy: Menstrual Bleeding Success Rates in Intent-
to-Treat Population
Bulk Symptoms
A fibroid-specific symptom questionnaire was used to assess
changes in three fibroid-related symptoms, pelvic pain, pelvic
discomfort, and urinary dysfunction. Success was defined as a
moderate or significant improvement for patients who entered
the study with moderate or severe symptoms, and/or no
worsening for patients who entered the study with no or mild
symptoms. Ninety-four of the 107 patients (88%) completed
the bulk symptom questionnaire at 6 months post-treatment.
All 13 patients with missing symptom data at 6 months for any
reason were counted as failures. Table 3 demonstrates that the
majority of UFE patients met the study criteria for success on all
three bulk-related symptoms.
This success was generally
achieved by 3 months. Eighty-four percent of the women met
the success criterion for at least one bulk symptom by 6
months.
Table 3 - Efficacy: Bulk Symptom Success Rates In Intent-to-
treat Population
Symptoms
6 Months
N/107 (%)
Pelvic Pain
83 (78%)
Pelvic Discomfort
80 (75%)
Urinary Dysfunction
75 (71%)
% Reduction From Baseline
6 Months
N/107 (%)
≥ 50%
69 (65%)
30-49%
15 (14%)
0-29%
7 (7%)
< 0%
10 (9%)
Lost to Follow-up
4 (4%)
Hysterectomy prior to 6 months
2 (2%)
