Care and use manual – Waters Amino Acid Analysis Liquid Chromatography Column User Manual
Page 2
[ Care and Use ManUal ]
2
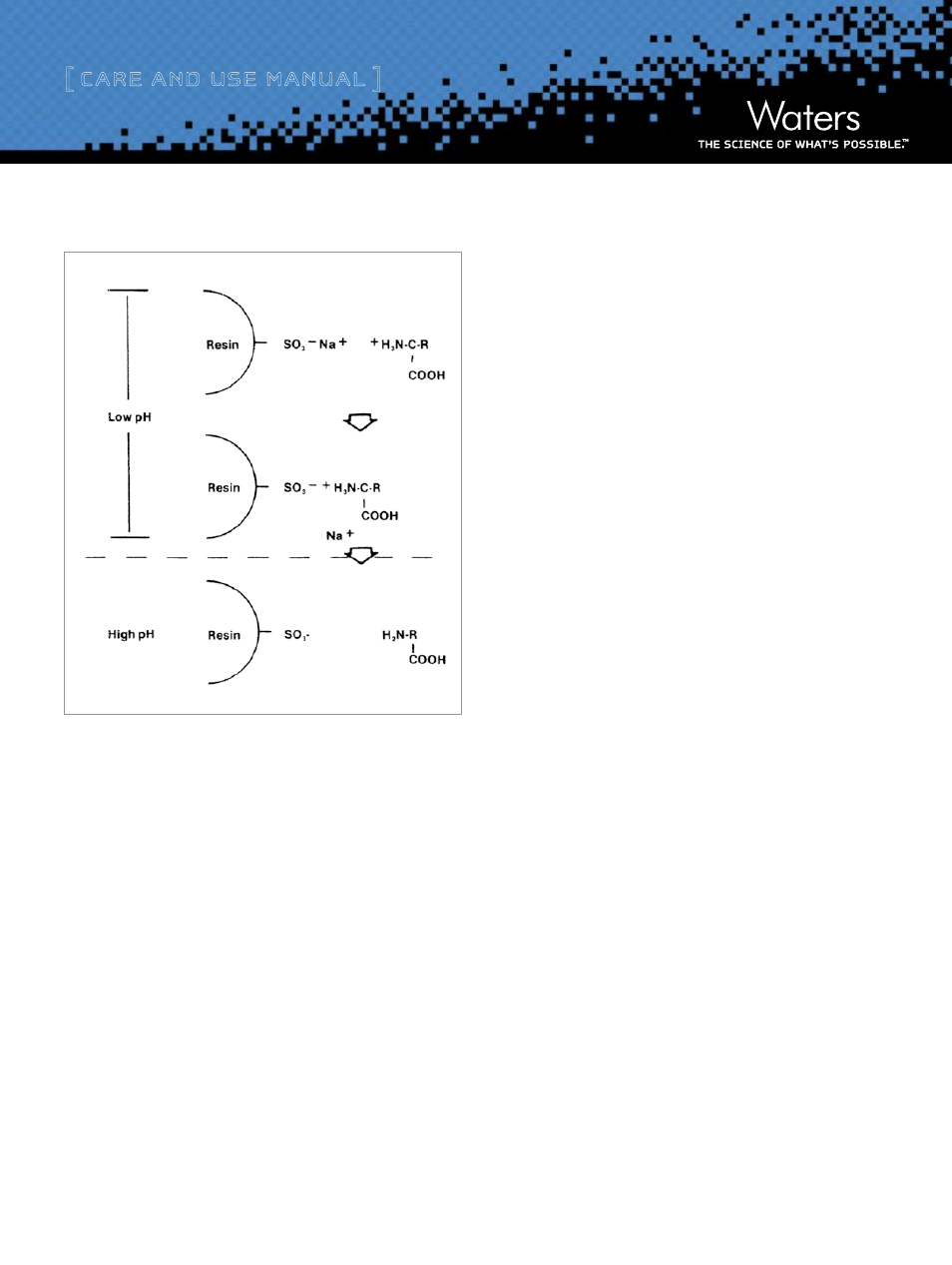
Figure 1: Ion Exchange Mechanism
CAUTION: Introduction of improperly prepared or contaminated buffers
or samples onto the resin bed could irreversibly poison the column.
Heavy metal ions can also poison the column. Column degradation will
be indicated by band broadening loss in resolution and/or change in
selectivity.
All reagents to be used for high performance liquid chromatography
(HPLC) analysis of amino acids must be of the highest purity. Water
should be distilled and deionized with a purity equivalent to that
obtained from a Milli-Q
®
water purifica¬tion system. Impure buffers will
lead to artifact peaks during analysis and shorten column lifetime.
There are two elution systems available for analysis:
1) Elution System 1 is the Waters Non-halide Buffer System. It is rec-
ommended for use where possible since it employs no potentially
corrosive halide ions.
2) Elution System 2 is a halide-containing buffer system and is rec-
ommended when maximum sensitivity is needed. Use of chloride
enhances orthopthalaldehyde (OPA) fluorescence by approximately
twofold as compared to the non¬halide elution system. The presence
of the halide ion requires immediate flushing of the system with
distilled water containing 0.2% sodium azide or 0.1% trifluoroacetic
acid before system shutdown. Allowing the halide buffers to sit
static in the pumps overnight will lead to corrosion.
a. elution system 1: Waters non-halide Buffer system
Buffer Composition
Buffer A-ES2
Sodium Citrate Dihydrate: 19.6 g
Phenol (Preservative):
1.0 g
HCI:
to pH 3.00
Total Volume:
1 L
Buffer B-ES1
Boric Acid:
1.5 g
Sodium Nitrate:
21.0 g
6M NaOH:
to pH 9.60
Total Volume:
1 L
NOTE: Do not add phenol to Buffer B; it will decompose at this pH.
b. elutIon system 2: halide Buffer system Buffer composition
Buffer Composition
Buffer A-ES2
Sodium Citrate Dihydrate: 19.6 g
Phenol (Preservative):
1.0 g
HCI:
to pH 3.00
Total Volume:
1 L
Buffer B-ES2
Boric Acid:
1.5 g
Sodium Chloride:
14.42 g
6M NaOH:
to pH 9.80
Total Volume:
1 L
