Care and use manual – Waters Protein-Pak HR Ion-Exchange Glass Columns User Manual
Page 9
[ Care and Use ManUal ]
Protein-Pak HR Ion-Exchanged Glass Columns
9
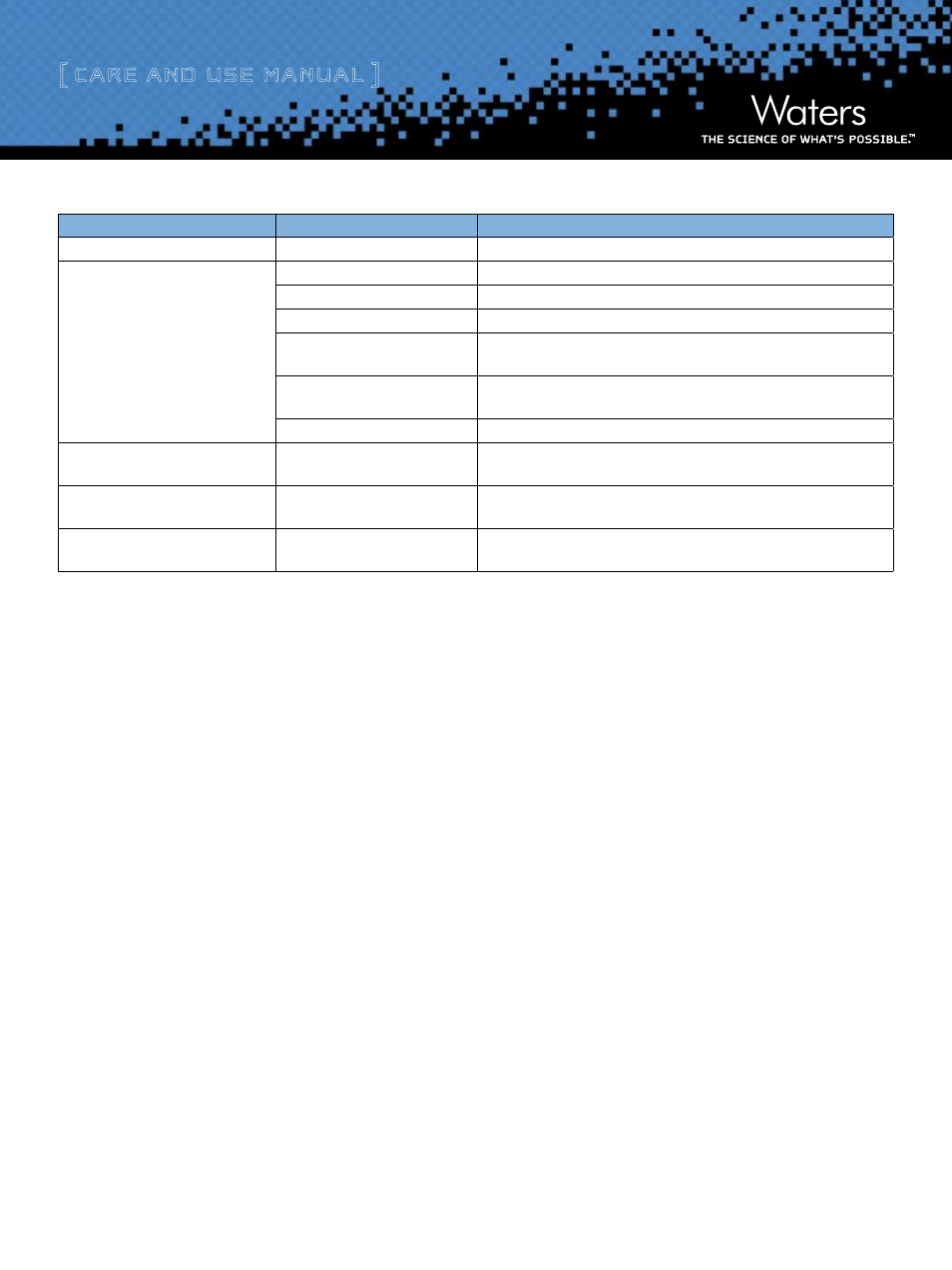
Problem
Cause
Solution
Noisy baseline
Gas in the mobile phase
Degas buffers or solvents prior to use. Sparge buffers during use.
Poor resolution
Sample overload
Reduce sample amount.
Flow rate too high
Reduce flow rate.
Gradient volume too small
Increase gradient volume.
Packed bed void
Tighten fine adjustment to eliminate void. Top off the column with appropriate
packing material.
Packing material fouled
Refer to packing material instructions for suitable cleaning agents (see Section
V, b).
Plugged column filters
Replace filter.
High backpressure
Fouled packing material
Plugged column filters
Flush with suitable cleaning agent. Replace filter.
Leakage at the compression screw
end fittings
Worn ferrules
Replace ferrules.
Leakage into the plastic shield or out of
the inlet connection assembly
Worn 0-rings
Replace 0-rings.
b) Column Cleaning and Regeneration
When column performance deteriorates because of sample
contamination, perform the following cleaning procedures to
regenerate the ion-exchange packing materials. Perform all washes
at half the recommended flow rates (refer to Section IV, Operating
Parameter Limits).
Part A
1. Wash the column with 4 column volumes of water to remove buffer
salts.
2. Flush the column with 4 column volumes of 0.1 M NaOH followed by 4
column volumes of water.
3. Pass 4 column volumes of 30% acetic acid (V/V) through the ion
exchanges, followed by 4 column volumes of water.
4. Flush 4 column volumes of 1 M NaCl to ensure that DEAE HR or Q HR
is in the chloride form and that SP HR or CM HR is in the sodium form.
5. Equilibrate with an appropriate buffer and repeat the functional test
outlined in Section V, a), Troubleshooting.
Part B
If column performance does not improve after Part A:
1. Incubate the column overnight with a proteolytic-enzyme solution
(1 mg/mL pepsin, trypsin, and papain) or nuclease solution (DNase)
under conditions for optimal enzyme activity.
2. After incubation, repeat Part A.
Part C
If you suspect lipid contamination:
1. Follow Part A procedures up to and including step 3.
2. Wash with 4 column volumes of 50% aqueous ethanol (absolute),
followed by 4 column volumes of water.
3. Equilibrate with an appropriate buffer (see Table 4), and repeat the
functional test outlined in Section V, a), Troubleshooting.
c. Storage Considerations
•
Store the columns in distilled water. It microbial contamination is
a concern, refrigerate at 4-6 °C or 39-43 °F. DO NOT FREEZE. For
long-term storage a 0.02% sodium azide solution or a 50% aqueous
ethanol solution may be used.
•
Return the column to its box with the end caps firmly in place for stor-
age. Allowing columns to dry out can result in poor chromatographic
performance.
Table 6: Typical Column Problems and Solutions
