Care and use manual, V. t roubleshooting, Xbridge protein beh c – Waters XBridge Protein BEH, C4, 300A, 3.5 µm Columns User Manual
Page 7: Waters preparative obd columns calculator
7
[ CARE AND USE MANUAL ]
XBridge Protein BEH C
4
, 300Å, 3.5, 5, 10 �m
§
Very strongly dependent on loading conditions
–
Limited by loading volume
–
Limited by diluent solvent strength
Waters Preparative OBD Columns Calculator
§
Convenient scale-up tool provides:
- Mass load scaling
- Gradient scaling with appropriate flow rate scale-up
and predicting volume consumption
- Calculations for split flow ratios for those using mass
spectrometer driven chromatography
- Focused gradient UPLC to preparative method transfer
AU
0.00
0.10
0.20
0.30
0.40
AU
0.00
0.10
0.20
0.30
0.40
AU
0.00
0.10
0.20
0.30
0.40
AU
0.00
0.10
0.20
0.30
0.40
Minutes
2.00
4.00
6.00
8.00
10.00
12.00
14.00
16.00
18.00
20.00
ACQUITY UPLC Protein BEH C
4
,
300Å, 1.7 µm
XBridge Protein BEH C
4
, 300Å, 3.5 µm
XBridge Protein BEH C
4
, 300Å, 5 µm
XBridge Protein BEH C
4
, 300Å, 10 µm
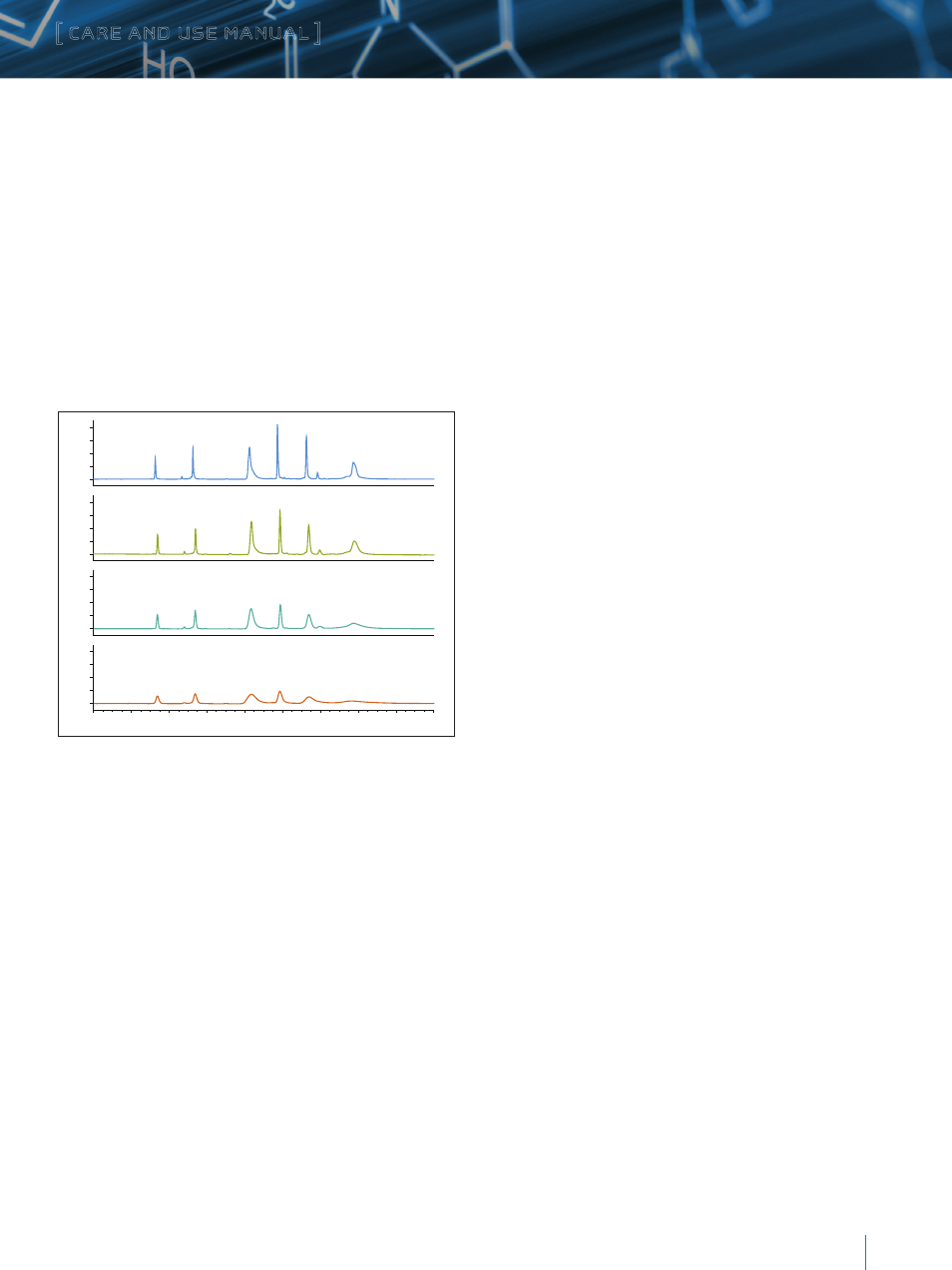
Figure 3: Separation of MassPREP Protein Standard Mixture on ACQUITY UPLC
Protein BEH C
4
, 300Å, 1.7 μm (Top), XBridge Protein BEH C
4
, 300Å, 3.5μm, XBridge
Protein BEH C
4
, 300Å, 5 μm, XBridge Protein BEH C
4
, 300Å, 10 μm using scaling
methods obtaining using Waters Preparative Chromatography OBD Calculator.
V. T ROUBLESHOOTING
The first step in systematic troubleshooting is comparison of the
column, in its current state, to the column when it was functioning
properly. The method suggested in Section I for measuring plate
count is an essential first step. This technique detects physical
changes to the packed bed and chemical changes in the bonded
phase surface. The two functional tests with the Peptide Standard
and the Protein Mixture may reveal more subtle changes in surface
chemistry that affect the application.
There are several common symptoms of change in the column.
1. An increase in pressure is often associated with lost
performance in the application. The first step in diagnosis
is to ensure that the elevated pressure resides in the column
rather than somewhere else in the system. This is determined
by measuring pressure with and without the column attached
to the instrument. If the system is occluded, the blockage
should be identified and removed. If the pressure increase
resides in the column, it is helpful to know whether the
problem was associated with a single injection or whether
it occurred over a series of injections. If the pressure
gradually built up, it is likely that the column can be cleaned
as described below (Section V). For future stability, it may
be useful to incorporate a stronger regeneration step in the
method. If a single sample caused the pressure increase,
it likely reflects particulates or insoluble components,
such as, lipids. Cleaning is still an option, but using the
more aggressive options. The sudden pressure increase
suggests that the user should consider some sample
preparation, such as filtration or high speed centrifugation.
2. Loss of retention can reflect a change in the column surface
chemistry. Before proceeding with diagnostic or corrective
measures, check that the mobile phases have been correctly
prepared and the correct method has been selected. Then
repeat the efficiency test and the functional peptide or
protein test. If both the small and large molecule test
show loss of retention, it is likely that a significant fraction
of the bonded phase has been lost, and the column will
require replacement. If the changes are small and reflected
only for some proteins, one of the cleaning procedures may
be effective.
3.
Change in peak shape, resolution, or relative retention of
peaks. Follow the same steps as for loss of retention (Symptom 2).
4. Carryover and memory effects are defined as the appearance
of the constituents of one sample in the next gradient analysis.
First determine whether the column or the system is the source
of carryover. Define a gradient method that includes an “inter-
nal gradient”. That is, the analytical gradient is repeated within
a single method. If the protein peaks appear in both gradients,
at the same time after start, the protein came from the column
in what is often described as a “memory effect”. If the protein
peaks only appear when an injection is made, they likely origi-
nate from adsorption to some system component. In that case,
follow the instrument manufacturer’s recommendations. Memory
effects as a source of carryover may be reduced or eliminated
in several ways. First, raising the temperature of the separation
