Care and use manual, Ii. column use, A. sample preparation – Waters Oligonucleotide Separation Technology XBridge OST C18 Columns User Manual
Page 4
[ CARE AND USE MANUAL ]
4
Table 2: Empty Column Volumes in mL (multiply by 10 for flush
solvent volumes)
Column Length
Internal
Diameter
mm
2.1 mm
4.6
50
0.2
1
100
0.4
2
150
0.5
2.5
c. Initial Column Efficiency Determination (See Section VI below)
It is recommended that an efficiency test be performed before using
your XBridge OST C
18
column for synthetic oligonucleotide
applications. Waters recommends using the same solutes and
conditions as found in the included Performance Test Chromatogram.
The efficiency test can be repeated to track the column performance
over time. Several chromatographic data acquisition software
packages (e.g., Waters Empower
™
2 Chromatography Data Software)
can determine the number of theoretical plates (N) from the test
chromatogram. Slight variations may be observed on two different
HPLC systems due to the quality of the connections, operating
environment, system electronics, reagent quality, column conditions,
and operator technique.
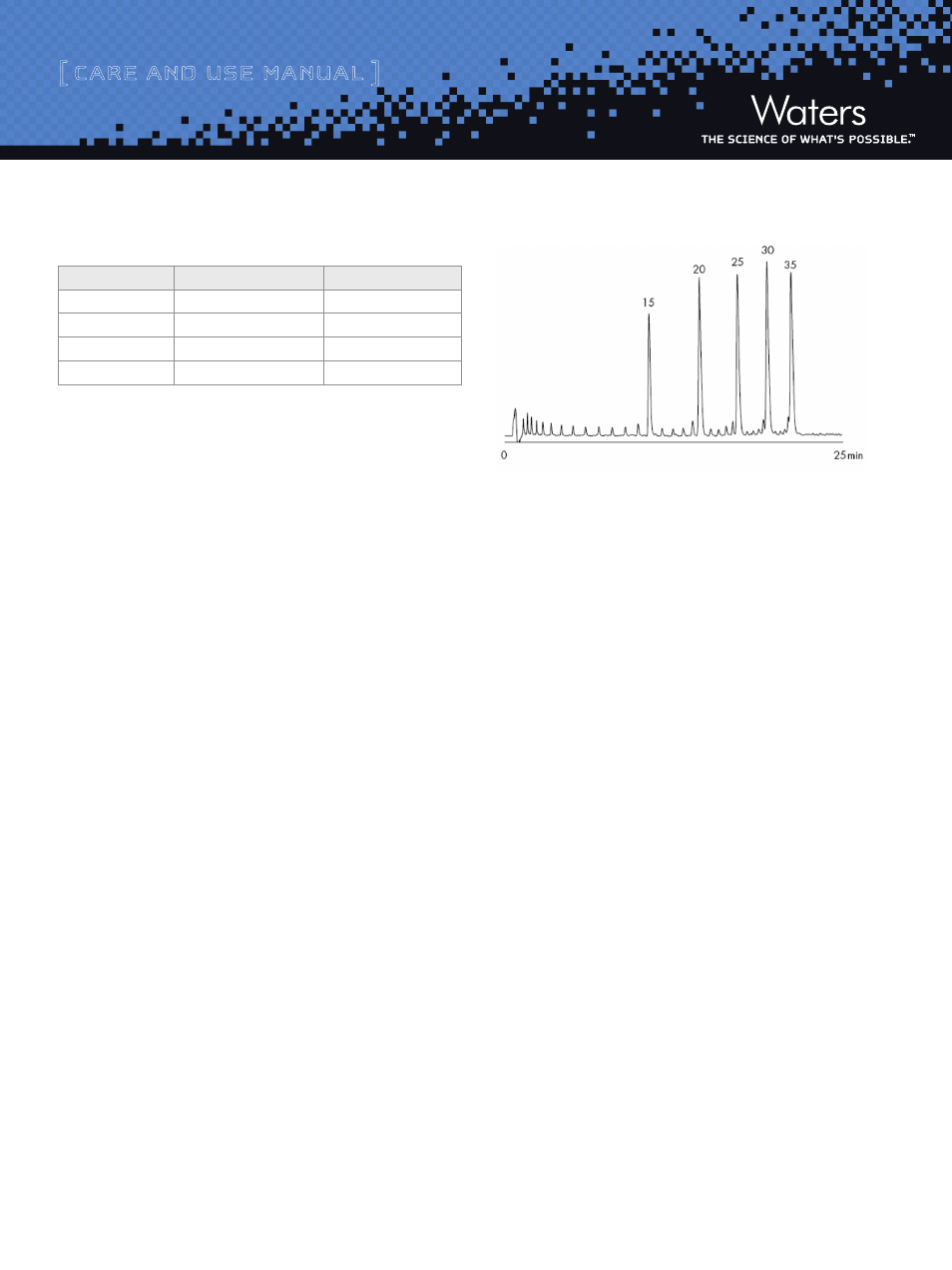
Column performance can be tested with the MassPREP OST Standard
(Part Number 186004135), a QC’d synthetic oligonucletoide
sample consisting of 15, 20, 25, 30 and 35mer deoxythymidine.
Approximately 0.1 nmol of each oligonucleotide was injected on the
Xbridge OST C
18
column. Refer to part number 715001677 for more
information on sample prep for the MassPREP OST Standard. Smaller
peaks eluting prior to the main peaks are failure, by-product sequences
from the synthesis.
Figure 6: Separation using the MassPREP OST Standard on
XBridge OST C
18
.
HPLC system:
Waters BioAlliance
™
2796, PDA Detector with micro UV cell
Sample Injected:
Approximately 0.1 nmol of MassPREP OST Standard
(Part Number 186004135) diluted in 0.1 M TEAA
Column:
Waters XBridge OST C
18
, 2.5 µm (2.1 x 50 mm)
Mobile Phases:
A: 0.1 M TEAA,
B: Acetonitrile / 0.1M TEAA, 20/80, v/v
Flow rate:
0.2 mL/min
Column Temp.:
60 ºC
Gradient delay:
0.45 mL
Gradient:
40 to 59.5% B in 26 minutes (8-11.9% acetonitrile, 0.15% acetonitrile
per minute)
Detection:
260 nm, 5 scans per second
II. COLUMN USE
To ensure the continued high performance of your XBridge OST C
18
columns, follow these guidelines:
a. Sample Preparation
1. Dissolve the detritylated synthetic oligonucleotide sample in mobile
phase A (e.g., 0.1 M TEAA). For example, a 0.05 - 0.2 µmole scale
synthesis can be prepared in 0.1 mL of 0.1 M TEAA. Proportionately
larger or smaller volumes of 0.1 M T EAA are required when
dissolving samples from different scale syntheses. Due to the
nature of gradient separations, relatively large volumes of sample
(in low organic strength eluent) can be injected and concentrated
onto the head of the column before beginning the gradient
elution program.
2. Samples must be completely in solution and free of particulates
before injection onto the column. Remove all particles from the
sample (Controlled Pore Glass Synthesis Support, etc.), which may
block the inlet column frit, increase the operating pressure, and
shorten the column life time. Sample contamination with high
concentration of salts and/or detergents may also interfere
with analysis.
