Appendix – LaMotte SMART 2 User Manual
Page 257
APPENDIX
Ammonia in water occurs in two forms: toxic unionized ammonia (NH
3
) and
the relatively non-toxic ionized form, ammonium ion (NH
4+
). This test
method measures both forms as ammonia-nitrogen (NH
3+
–N) to give the total
ammonia-nitrogen concentration in water. The actual proportion of each
compound depends on temperature, salinity, and pH. A greater concentration
of unionized ammonia is present when the pH value and salinity increase.
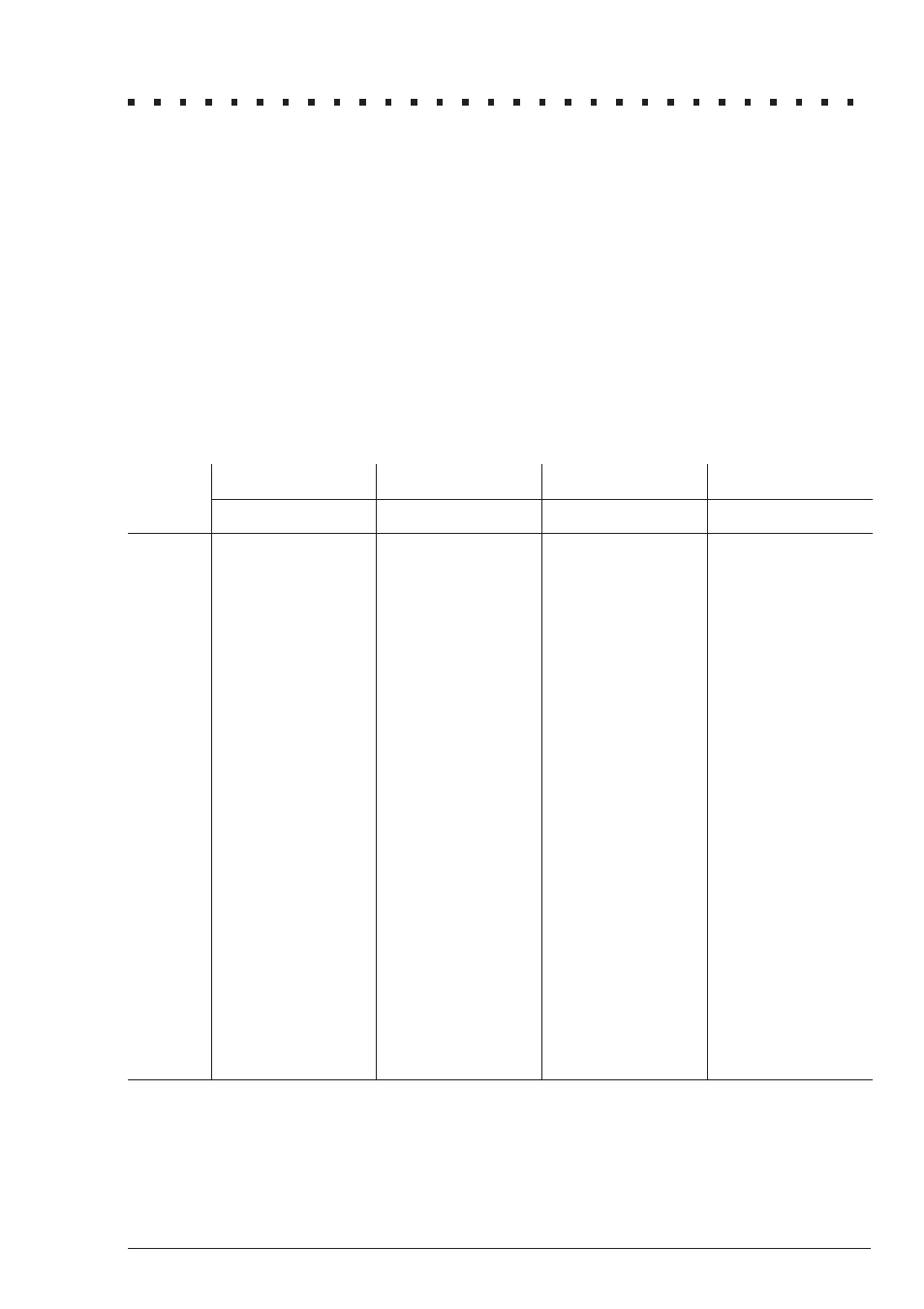
1.
Consult the table below to find the percentage that corresponds to the
temperature, pH, and salinity of the sample.
2.
To express the test result as ppm Unionized Ammonia Nitrogen
(NH
3
–N), multiply the total ammonia-nitrogen test result by the
percentage from the table.
3.
To express the test result as ppm Ammonia Nitrogen (NH
3+
–N), subtract
the unionized ammonia-nitrogen determined in step 2 from the total
ammonia-nitrogen.
10
°C
15
°C
20
°C
25
°C
pH
FW
1
SW
2
FW
SW
FW
SW
FW
SW
7.0
0.19
—
0.27
—
0.40
—
0.55
—
7.1
0.23
—
0.34
—
0.50
—
0.70
—
7.2
0.29
—
0.43
—
0.63
—
0.88
—
7.3
0.37
—
0.54
—
0.79
—
1.10
—
7.4
0.47
—
0.68
—
0.99
—
1.38
—
7.5
0.59
0.459
0.85
0.665
1.24
0.963
1.73
1.39
7.6
0.74
0.577
1.07
0.836
1.56
1.21
2.17
1.75
7.7
0.92
0.726
1.35
1.05
1.96
1.52
2.72
2.19
7.8
1.16
0.912
1.69
1.32
2.45
1.90
3.39
2.74
7.9
1.46
1.15
2.12
1.66
3.06
2.39
4.24
3.43
8.0
1.83
1.44
2.65
2.07
3.83
2.98
5.28
4.28
8.1
2.29
1.80
3.32
2.60
4.77
3.73
6.55
5.32
8.2
2.86
2.26
4.14
3.25
5.94
4.65
8.11
6.61
8.3
3.58
2.83
5.16
4.06
7.36
5.78
10.00
8.18
8.4
4.46
3.54
6.41
5.05
9.09
7.17
12.27
10.10
8.5
5.55
4.41
7.98
6.28
11.18
8.87
14.97
12.40
1
Freshwater data from Trussel (1972).
2
Seawater values from Bower and Bidwell (1978).
Salinity for Seawater values = 34% at an ionic strength of 0.701m.
Smart2 TEST PROCEDURES 2.04
Appendix 1/2
