Care and use manual, Iv. column use – Waters Protein Separation Technology ACQUITY UPLC BEH300, C4, 1.7 µm Columns User Manual
Page 5
[ Care and Use ManUal ]
Protein Separation Technology ACQUITY UPLC BEH300, C
4
, 1.7
�m
5
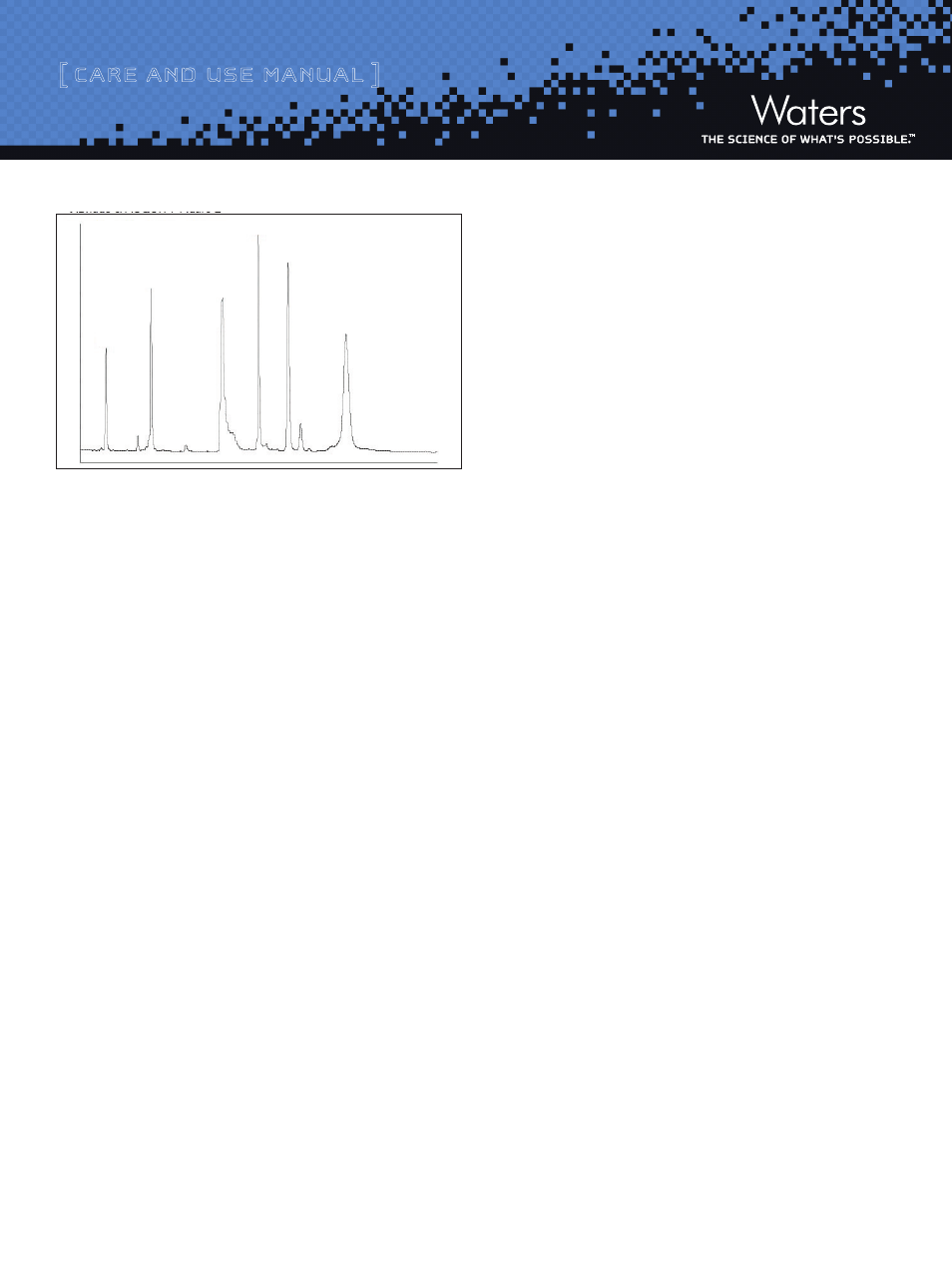
Figure 2: Typical Protein Test Mixture Chromatogram
This chromatogram is typical of the results obtained in Waters
Laboratories with the method described above, using a 2.1 x 50 mm
column. The retention times will double and triple for 100 mm and
150 mm columns respectively. The exact results observed in any
laboratory will depend on the instrument in use. Similar results can
only be expected with a Waters ACQUITY UPLC System. This test is
exceptionally valuable for monitoring the life of the column and for
troubleshooting separation difficulties that may arise.
IV. CoLuMn use
To ensure the continued high performance of ACQUITY UPLC
BEH300, C
4
, 1.7 µm columns, follow these guidelines:
a. Sample Preparation
1. Sample impurities often contribute to column contamination.
Samples should be free of particles before injection into the
system.
2. It is preferable to prepare the sample in the operating mobile
phase or a mobile phase that is weaker (less organic modifier)
than the mobile phase for the best peak shape and sensitivity.
3. If the sample is not dissolved in the mobile phase, ensure that
the sample, solvent and mobile phases are miscible in order to
avoid sample and/or buffer precipitation.
4. Filter sample with 0.2 μm filters to remove particulates. If
the sample is dissolved in a solvent that contains an organic
modifier (e.g., acetonitrile, methanol, etc.) ensure that the
filter material does not dissolve in the solvent. Contact the
filter manufacturer with solvent compatibility questions.
Alternatively, centrifugation for 20 minutes at
12-50,000 g, followed by the transfer of the supernatant liquid
to an appropriate vial, could be considered.
1
2
3
4
5
6
1 - RNase A
2 - Cyt. C
3 - BSA
4 - Myoglobin
5 - Enolase
6 - Phosphorylase b
