Application, Description – M&C TechGroup PMA 100 Operator's manual User Manual
Page 5
9-3.12-ME
M&C Products Analysentechnik GmbH
- 5 -
6. Application
The transducer of the PMA100 works at a stable tempe-
rature of +55
°
C. Therefore the analyser is suitable for con-
tinuous measurements of oxygen concentrations in particle-
free and dry sample gases.
Safe operation, reliability and minimized maintenance are
the characteristic of the PMA100.
The operation of the instrument is based upon the principle
of the magneto-dynamic cell which is the most accurate
and reliable cell for determining the oxygen content in gas
mixtures in a range of 0 to 100 Vol.-%.
The patented M&C measuring cell has been improved in
order to achieve stability, minimum drift of temperature and
extremely fast response time. Due to this fast response
time and the negligible cross-sensitivity from other gases
the PMA100 is applicable in a wide range of processes,
like:
• monitoring of flue gases,
• inerting installations,
• fermentation processes,
• process- and lab-measurements, etc.
7. Description
7.1
Measuring principle
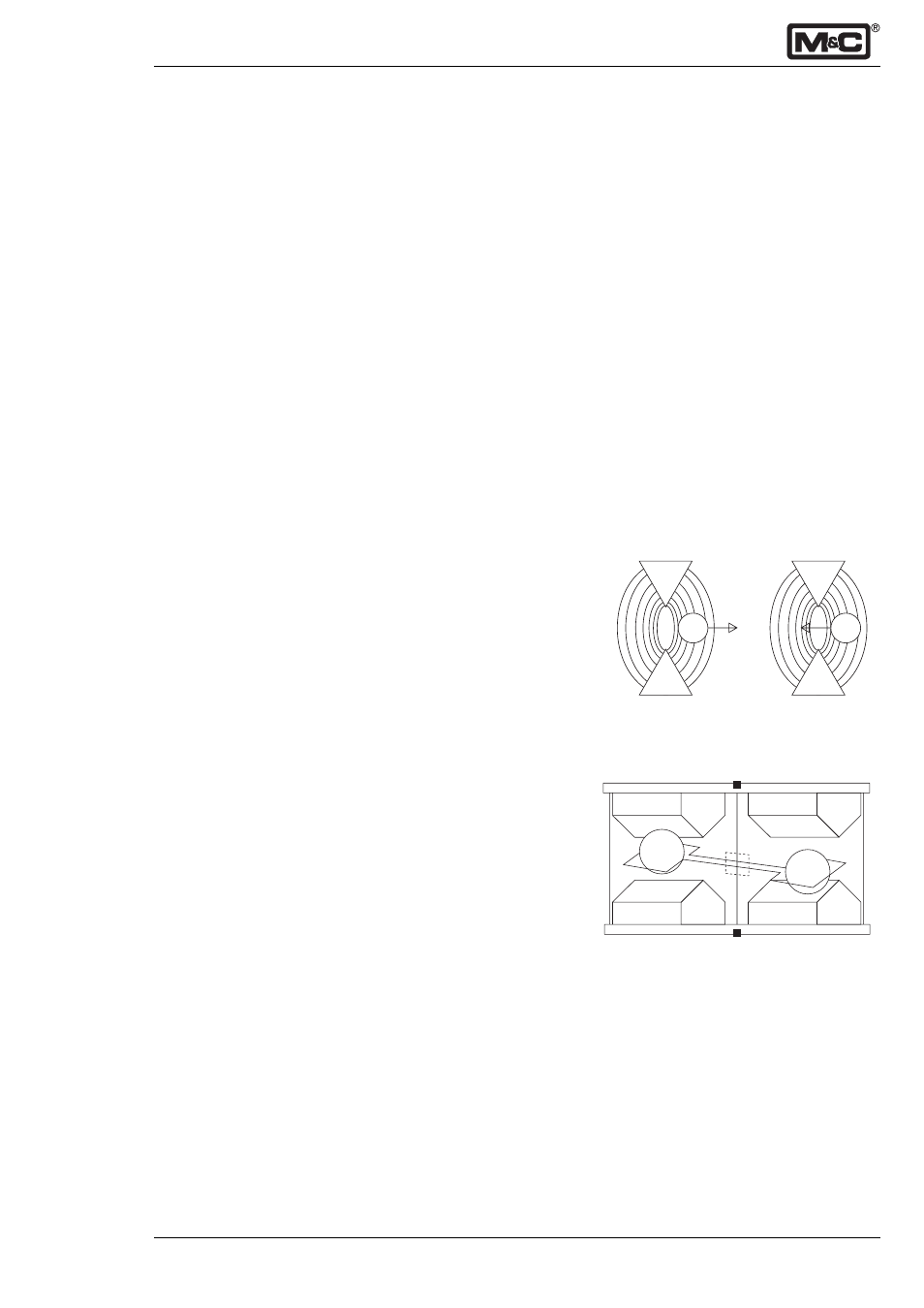
The paramagnetic susceptibility of oxygen is significantly
greater than that of other common gases, and for this
reason the molecules of oxygen are attracted much more
strongly by a magnetic field than the molecules of other
gases. Most of the other gases are slightly diamagnetic,
e.g. the molecules are then repelled by a magnetic field.
The principle of the magneto-dynamic cell is based upon
Faraday’s method of determining the magnetic suscepti-
bility of gas. The cell consists of two nitrogen-filled quarts
spheres arranged in the form of a dumb bell. A single turn
of platinum wire is placed around the dumb bell which is
suspended in a symmetrical non-uniform magnetic field.
When the surrounding gas contains oxygen, the dumb bell
spheres are pushed out of the magnetic field by the change
in the field which is caused by the relatively strong para-
magnetic oxygen. The torque acting on the dumb bell will
be proportional to the paramagnetism of the surrounding
gas and consequently it can be used as a measure of the
oxygen concentration.
The distortion of the dumb bell is sensed by a light-beam
and projected on a mirror attached to the dumb bell where-
of it is reflected to a pair of photo cells (Fig. 3). When both
photo cells are illuminated equally the output will be zero.
The output from the photo cells is connected to an amplifier,
which in turn is fed to the feedback coil of the measuring
cell. If the oxygen content of the gas sample changes,
Nitrogen=Diamagnetic Oxygen=Paramagnetic
Fig. 1:
Magnetic susceptibility of gases
1 : Quarts sphere dumb bell
2 : Platinum wire
3 : Mirror
4 : Magnetic pole pieces
Fig. 2:
The measuring cell in theory
N
S
N
S
N
2
O
2
➡➡
1
3
2
4
