LaMotte LTC3000wi Turbidity & Chlorine Lab Meter User Manual
Page 18
meters using different wavelengths should not be expected to give
the same result because the absorbance spectrum of natural water is
usually not identical to the absorbance spectrum of chloroplatinate/
cobalt standards. The reading that the meter displays is a correlation
between the color of the sample water and the color standards at a fi xed
wavelength. The correlation and reading will change as the wavelength
changes.
TAKING COLOR WATER SAMPLES
Samples should ideally be collected in glass containers. Perform the
analysis soon after sampling since the color may change with time. For
true color determinations, remove turbidity by fi ltration or centrifugation.
SAMPLE DILUTION TECHNIQUES
If a test result is out of the range of the meter, it must be diluted. The
test should then be repeated on the diluted sample. The following table
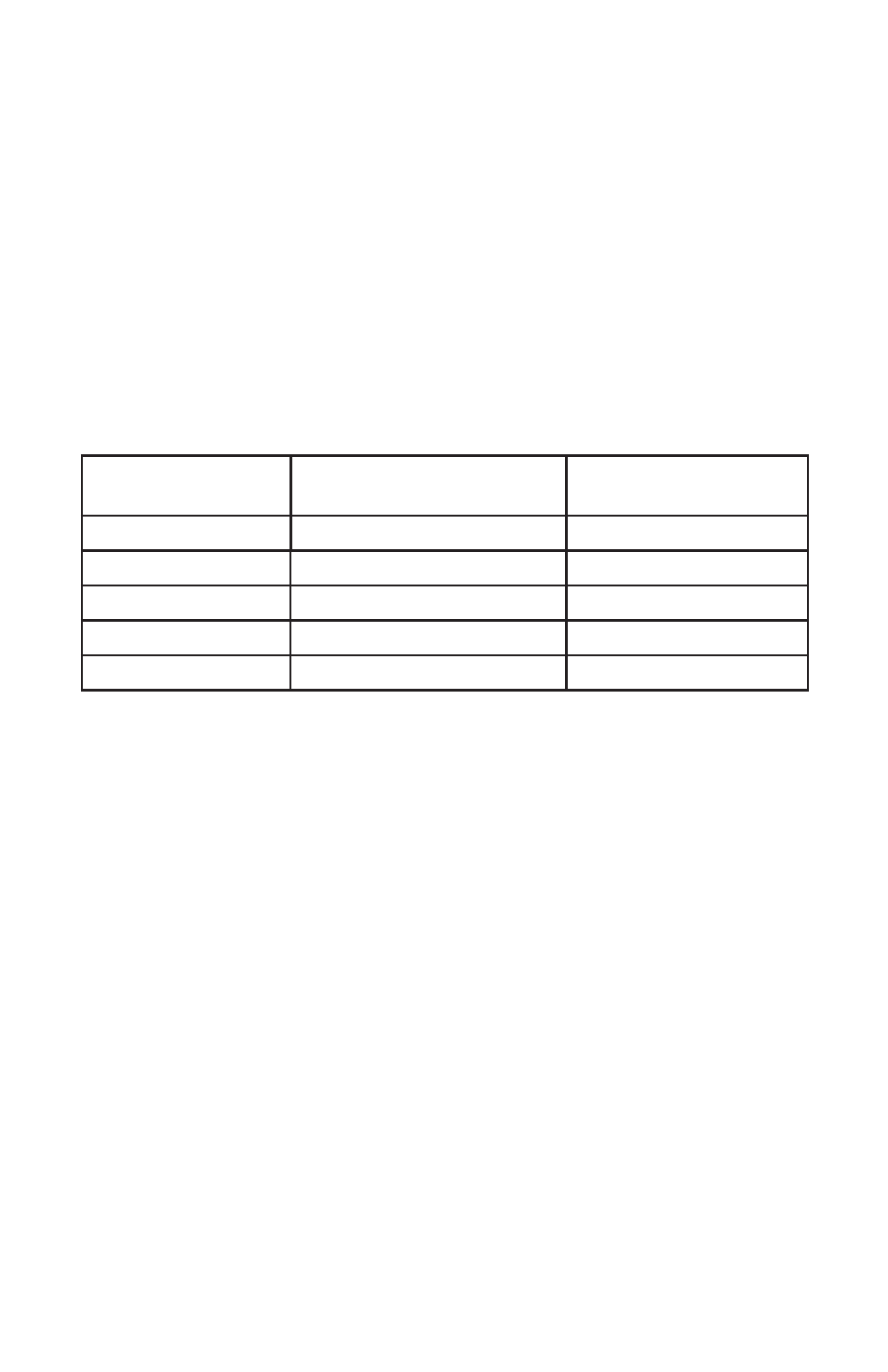
gives quick reference guidelines for dilutions of various proportions.
Amount of Sample Deionized Water to Bring
Final Volume to 10 mL
Multiplication Factor
10 mL
0 mL
1
5 mL
5 mL
2
2.5 mL
7.5 mL
4
1 mL
9 mL
10
0.5 mL
9.5 mL
20
All dilutions are based on a fi nal volume of 10 mL, so several dilutions
will require small volumes of the water sample. Graduated pipets should
be used for all dilutions. If volumetric glassware is not available, dilutions
can be made with the colorimeter tube. Fill the tube to the 10 mL line
with the sample and then transfer it to another container. Add 10 mL
volumes of deionized water to the container and mix. Transfer 10 mL of
the diluted sample to the colorimeter tube and follow the test procedure.
Repeat the dilution and testing procedures until the result falls within
the range of the calibration. Multiply the test result by the dilution factor.
For example, if 10 mL of the sample water is diluted with three 10 mL
volumes of deionized water, the dilution factor is four. The test result of
the diluted sample should be multiplied by four.
18
