Care and use manual, Iv. qcrm testing – Waters Preparative Chromatography Mix Standard User Manual
Page 3
[ CARE AND USE MANUAL ]
Preparative Chromatography Mix
3
IV. QCRM TESTING
The use of reference standards for QCRM testing should allow the
analyst to track important instrument analytical parameters such as
peak width, peak area, retention time, and peak resolution. Each of
these important parameters can be tracked and evaluated using control
charts. The use of a high quality reference standard allows the analyst
to reliably measure and track these parameters.
QCRM testing should be performed on a regular basis for each
instrument/analyst combination or instrument per test method. The
data should be collected and entered into a control chart allowing
the analyst to evaluate the system performance over time. The use of
performance control charts has been a staple of analytical chemistry
quality control. The most common form of the control charting is to
track the analytical results and statistically analyze the data to a 99%
(3 standard deviations) or 95% confidence interval (2 standard
deviations) confidence interval around the mean of the data to
establish upper control limits (UCL) and lower control limits (LCL).
The initial criteria to establish a mean, standard deviation and
control limits involves analyzing a reference material a minimum
of 7 times to establish an initial estimate of precision and bias. This
provides the analyst with sufficient data to be statistically valid. The
analyses should be carried over the course of several days to provide
a more realistic view of the system variability. The frequency
of analyzing system performance will be dependent on the stability
of the analysis and the analytes. QCRM should always be evaluated
after maintenance has been performed, or when changes to the
system or analytical procedure have been made.
The example in Table 1 uses retention time monitoring to establish
a set of control limits for the purpose of monitoring on-going
system performance.
Table 1: Reference Standard Retention Time Data Example
Analysis Peak
Retention Time (mins)
1
7.10
2
7.11
3
7.12
4
7.09
5
7.08
6
7.10
7
7.11
8
7.13
9
7.10
10
7.11
Mean 7.11
Standard Deviation 0.0136
LCL 7.08
UCL 7.13
The standard reference material was analyzed 10 times yielding
the above retention times. The mean retention time and standard
deviation were calculated and from this the UCL and LCL limits
were determined. The control limits represent a 95% confidence
interval (2 standard deviations) for the data. The control chart
in Figure 2 was then produced to establish that the instrument
retention times are in control.
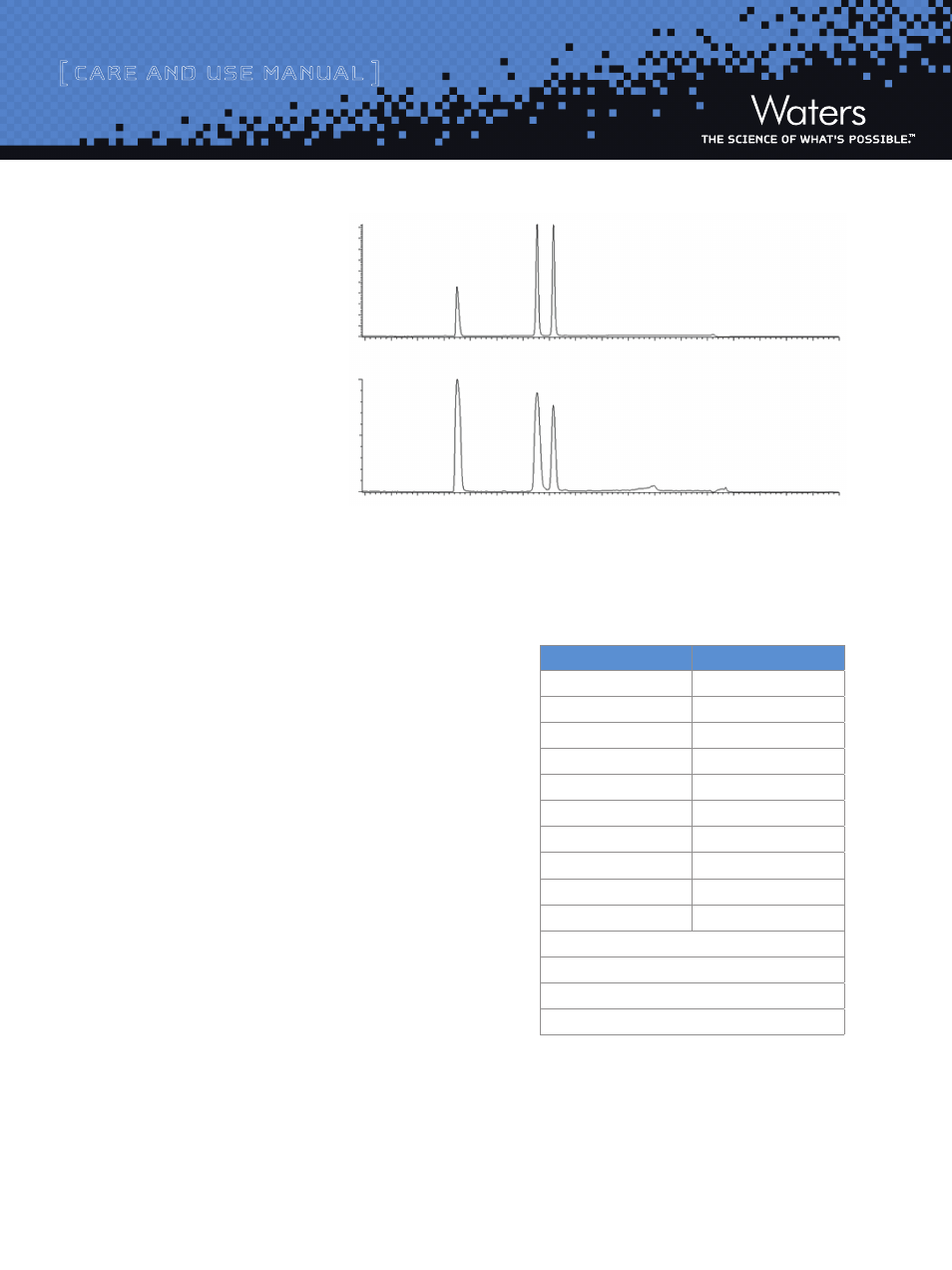
Figure 2 shows an example of the
chromatography obtained for the
preparative mix via UV and MS
when the method in Figure 1 is
using an XSelect
™
CSH
C
18
, 5 µm,
19 x 50 mm.
1. Diphenhydramine
2. Flavone
3. Diclofenac
220 nm
TIC; ES +
1
2
3
1
-1
0.0
2.0e-1
4.0e-1
6.0e-1
8.0e-1
AU
%
99
2
3
4
5
6
7
8
9
10 min
1
2
3
4
5
6
7
8
9
10 min
