Care and use manual – Waters Protein-Pak Hi Res IEX Columns User Manual
Page 8
8
[ CARE AND USE MANUAL ]
Protein-Pak Hi Res IEX Columns and Standards
At the time of manufacture, tracking and quality control
information will be downloaded to the eCord. Storing this
information on the chip will eliminate the need for a paper
Certificate of Analysis. Once the user installs the column, the
software will automatically download key parameters into a
column history file stored on the chip. In this manual, we explain
how the eCord will provide a solution for easily tracking the
history of the columns, reduce the frustration of paperwork trails,
and give customers the reassurance that a well performing column
is installed onto their instruments.
Figure E. eCord Inserted into Side of Column Heater.
b. Installation
Install the column into the column heater. Plug the eCord into
the side of the column heater. Once the eCord is coupled to the
reader, the overall column usage information will be available
in the ACQUITY
®
console, allowing the user to access column
information on their desktop.
c. Manufacturing Information
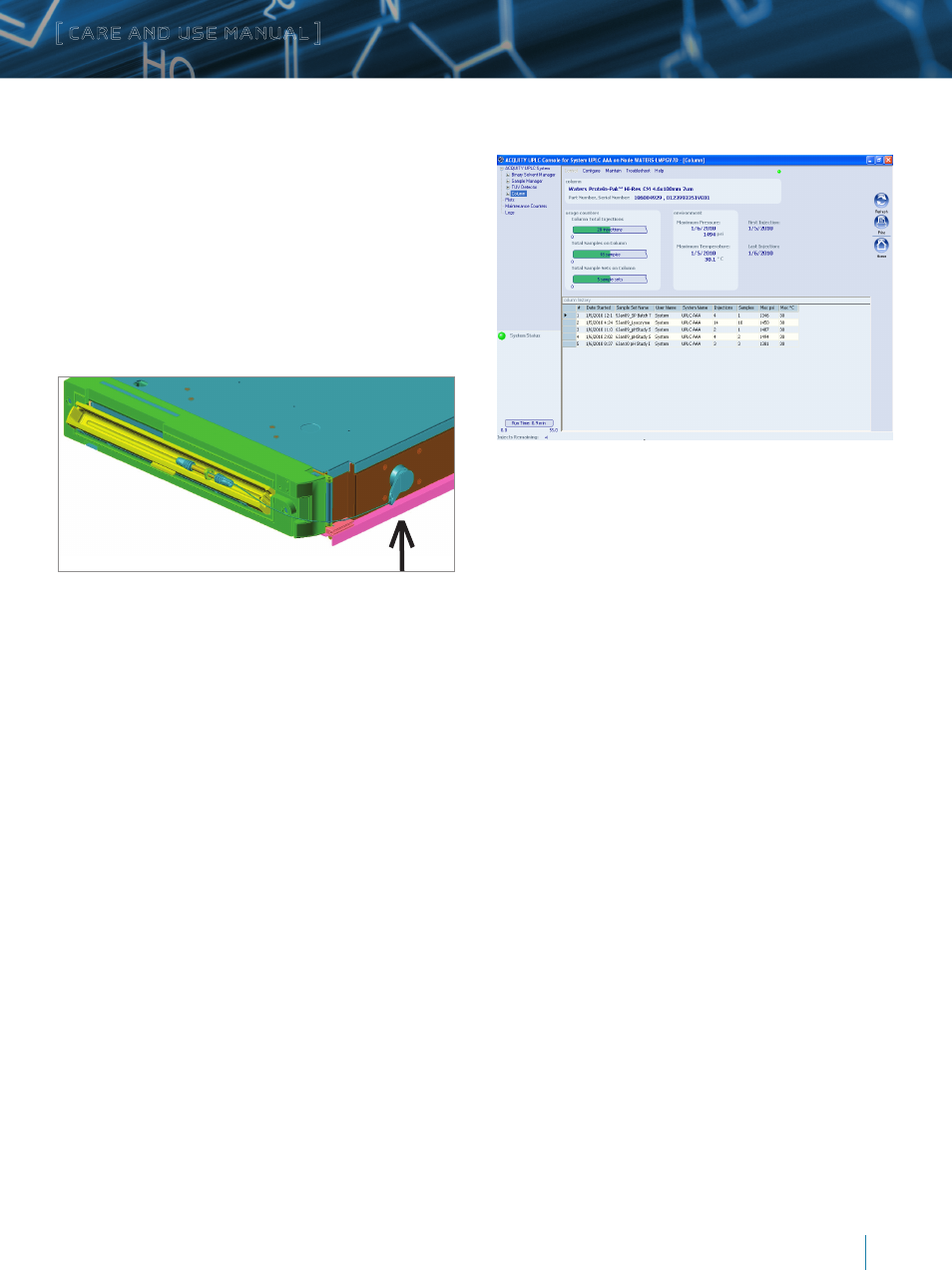
The eCord chip provides the user with an overview of the bulk
material QC test results. The eCord chip provides the user with QC
test conditions and results on the column run by the manufacturer.
The information includes mobile phases, running conditions and
analytes used to test the columns. In addition, the QC results and
acceptance is placed onto the column.
d. Column Use Information
The eCord chip provides the customer with column use data. The
top of the screen identifies the column including chemistry type,
column dimensions and serial number. The overall column usage
information includes the total number of samples, total number
of injections, total sample sets, date of first injection, date of last
injection, maximum pressure, and temperature. The information
also details the column history by sample set including date
started, sample set name, user name, system name, number of
injections in the sample set, number of samples in the sample set,
maximum pressure, and temperature in the sample set and if the
column met basic system suitability requirements.
