10 explanation of alkaline direct distillation, 4 .10 explanation of alkaline direct distillation – BUCHI KjelSampler K-377 User Manual
Page 40
4 Description of function
40
K-375/376/377 Operation Manual, Version B
4 .10 Explanation of alkaline direct distillation
As an example, the protein content in milk samples can be determined by direct distillation. This
quick method is based on the fact that milk releases ammonia when boiled in an alkaline solution.
Most of this ammonia is produced by the rapid hydrolysis of proteins containing glutamine and aspar-
agine. This decomposition is completed within a few minutes. An additional quantity of ammonia,
although small, is released through the complete transformation of other amino-acids. This second
reaction occurs very slowly however, and does not interfere with the quick method. This fact permits
an experimental determination of the ratio of total nitrogen or protein to ammonia nitrogen which is
released by boiling in an alkaline solution. Once the resulting conversion factor is determined, a series
of analysis can be carried out for control purposes without the time-consuming digestion step. The
overall analysis is reduced to the following steps:
• Sample addition
• Dilution
• Alkalisation
• Distillation
• Titration
• Calculation
A determination can be completed in approx. 10 minutes according to this procedure. All working
conditions chosen for the experimental determination of the conversion factor must be strictly
adhered to during sample measurements.
For details on the application procedure, please contact your local BUCHI representative.
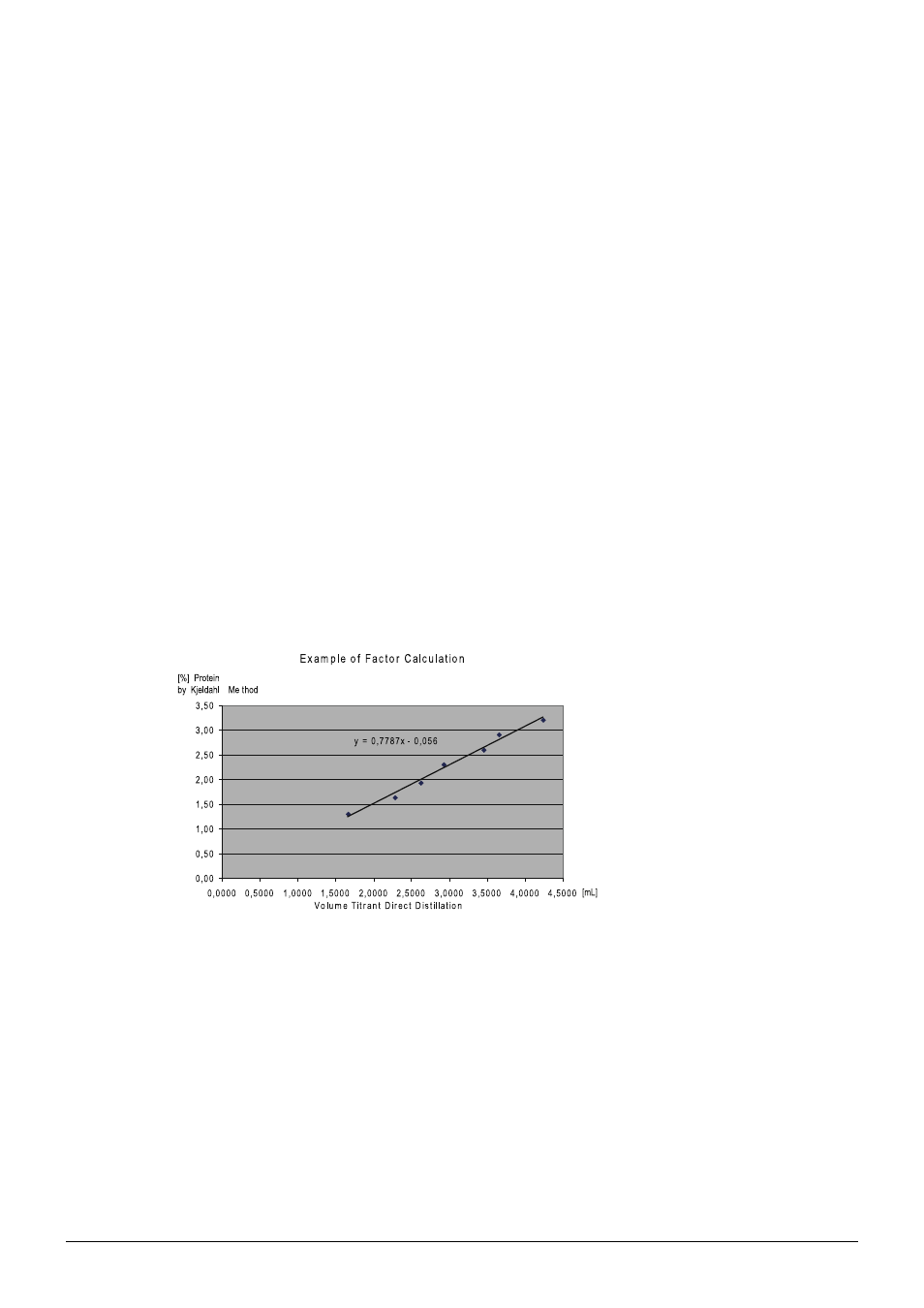
Determination of the conversion factor and the regression factor:
Fig. 4.8: Example of factor calculation
