Sample preparation, First-dimension separation: ief, Detection – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 6: Image acquisition, analysis, and spot cutting
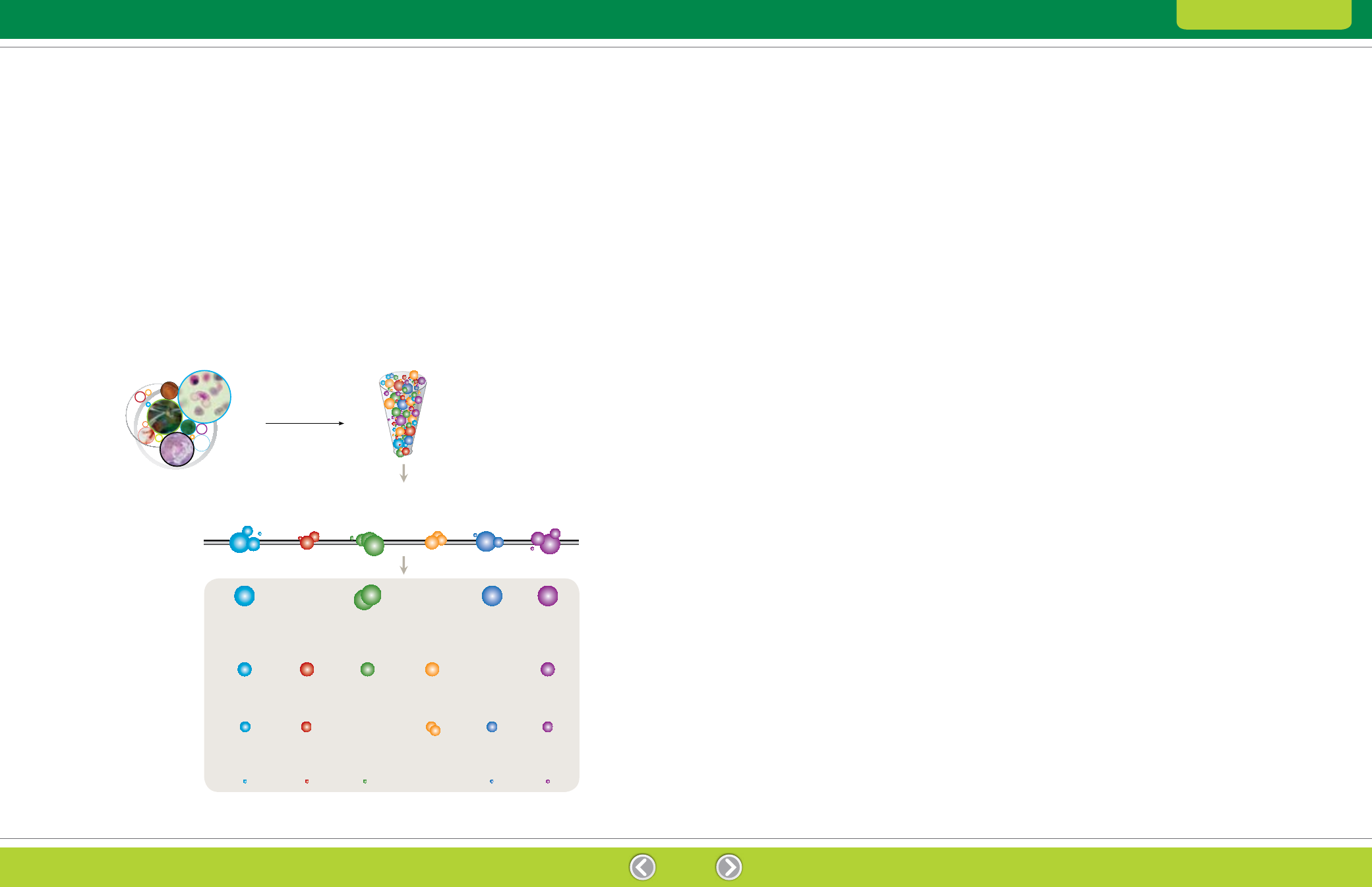
Fig. 1.2. 2-D electrophoresis. Protein spots result from two separations: first by pI (IEF) and then by size (SDS-PAGE).
Sample Preparation
Low pH
High pH
High MW
Low MW
Second Dimension
SDS-PAGE,
separation by MW
First Dimension
Isoelectric focusing (IEF), separation by pl
8
9
2-D Electrophoresis Guide
Theory and Product Selection
Chapter 1: Overview of Two-Dimensional Electrophoresis
Sample Preparation
Effective sample preparation is key for the success
of the experiment. The sample dictates the type of
extraction technique used, and the solubility, charge,
and pI of the proteins of interest affect the method
of solubilization. The protein fraction used for 2-D
electrophoresis must be solubilized in a denaturing
solution of low ionic strength; this solution cannot
contain components that alter protein size or charge.
Sample preparation also involves optional steps to
deplete abundant proteins, reduce the complexity
of the protein mixture, or select a subproteome of
interest. Details and recommendations for sample
preparation can be found in Chapter 2.
First-Dimension Separation: IEF
In 2-D electrophoresis, the first-dimension separation
step is IEF. Proteins are separated electrophoretically
on the basis of their pI, the pH at which a protein
carries no net charge. For general proteome analysis,
IEF is best performed in an immobilized pH gradient
Detection
Proteins separated in gels are usually not visible to
the naked eye and must, therefore, be either stained
or labeled for visualization. Several factors determine
the best choice of staining method, including desired
sensitivity, linear range, ease of use, expense, and the
type of imaging equipment available. There is no ideal
universal stain. Sometimes proteins are detected after
transfer to a membrane support by western blotting.
These topics are discussed in Chapters 5 and 6.
Image Acquisition, Analysis, and Spot Cutting
The ability to collect data in digital form is one of the
major factors that make 2-D gels a practical means
of collecting proteome information. It allows the
unbiased comparison of samples and gels, transfer of
information among research groups, and cataloguing
of data. Many types of imaging devices interface with
software designed specifically to collect, interpret,
and compare proteomics data.
Once interesting proteins are selected by differential
analysis or other criteria, the proteins can be excised
from gels and identified by mass spectrometry.
The ExQuest
™
spot cutter, which can be operated
independently or programmed to run from PDQuest
™
software, automatically cuts selected protein spots
from gels with precision and deposits them into the
wells of microplates.
Imaging equipment, software, and the ExQuest spot
cutter are discussed in Chapter 6.
Protein Digestion and Identification by
Mass Spectrometry
The excised gel plugs are destained and enzymatically
digested (usually with trypsin) in preparation for
identification by mass spectrometry. The use of mass
spectrometry for precise mass and partial sequence
determination, coupled with the availability of protein
sequence databases, has made high-throughput
protein identification possible. An overview of this
process is provided in Chapter 7.
(IPG) strip and under conditions aimed at completely
denaturing and solubilizing all the proteins in the
sample (as opposed to native IEF, which aims to
preserve native structures and activities). Chapter 3
discusses IEF.
Second-Dimension Separation: SDS-PAGE
The second-dimension separation step is SDS-PAGE,
where the proteins already separated by IEF are further
separated by their size. Prior to second-dimension
separation, an equilibration step is applied to
the IPG strip containing the separated proteins.
This process reduces any disulfide bonds that may
have re-formed during the first dimension and alkylates
the resultant sulfhydryl groups. Concurrently, the
proteins are complexed with SDS for separation on
the basis of size. Following electrophoretic separation
on a slab gel, the result is a two-dimensional array
of separated protein “spots” (Figure 1.2). Second-
dimension SDS-PAGE is discussed in Chapter 4.
