Removal of interfering substances, General considerations, Nucleic acids (dna and rna) – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 11: Dnase requires magnesium ions for activity, Or mgcl
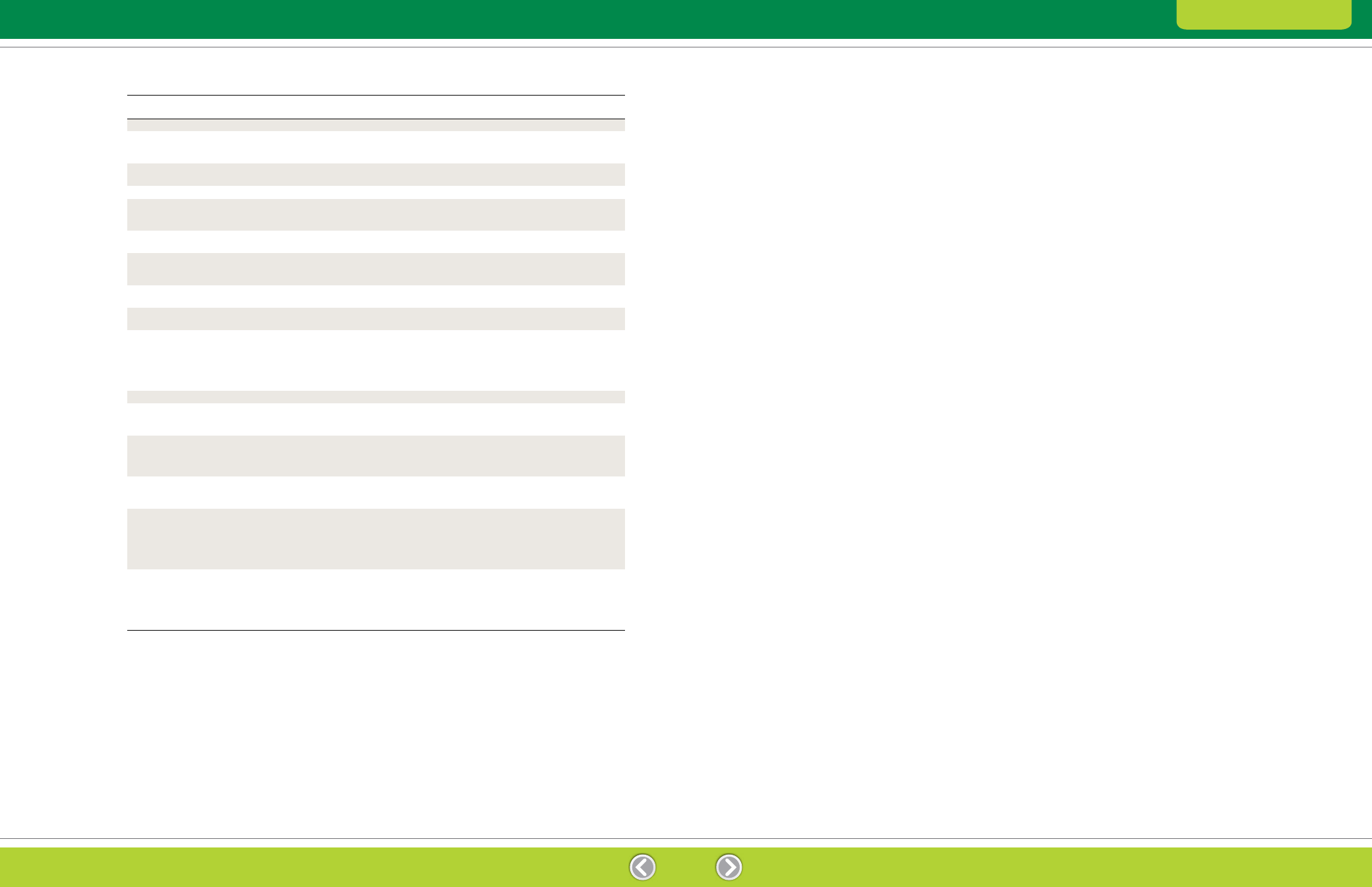
Table 2.2. Summary of compounds used in 2-D electrophoresis sample solutions. Refer to Ordering Information (Appendix C) for catalog
numbers and details of options available for purchase.
Role in
Concentration
Compound or product
Solution
Range
Comments
Urea
Chaotrope
5–9.5 M
Present during first-dimension IEF
Thiourea
Chaotrope
2 M
Used with urea, usually in the combination 7 M urea, 2 M thiourea;
more effective than urea alone for solubilizing hydrophobic or high
molecular weight proteins
CHAPS
Detergent
1–4% (w/v)
Zwitterionic detergent that may enhance protein solubility with minimal
disruptive effect on 2-D electrophoresis (Perdew et al. 1983)
CHAPSO
Detergent
1–4% (w/v)
Zwitterionic detergent similar to CHAPS
NP-40
Detergent
0.5–1% (w/v)
Neutral detergent originally used in 2-D electrophoresis (O’Farrell 1975,
Görg et al. 1988); its use has been largely superseded by CHAPS
(Görg et al. 2004)
Triton X-100
Detergent
0.5–1% (w/v)
Neutral detergent similar to NP-40 also used for 2-D sample
preparation (Kawaguchi and Kuramitsu 1995)
SB 3-10
Detergent
1–2% (w/v)
Zwitterionic detergent shown in some cases to give better solubilization
than CHAPS; insoluble in higher concentrations of urea and generally
used with 5 M urea, 2 M thiourea (Rabilloud et al. 1997)
ASB-14
Detergent
1–2% (w/v)
Zwitterionic detergent developed for solubilization of membrane
proteins to be analyzed by 2-D electrophoresis (Chevallet et al. 1998)
ASB-C8Ø
Detergent
1–2% (w/v)
Zwitterionic detergent developed for solubilization of membrane
proteins to be analyzed by 2-D electrophoresis (Chevallet et al. 1998)
Sodium dodecyl sulfate
Detergent
Up to 2% (w/v)
Anionic detergent widely used in sample preparation for
(SDS)
during sample
electrophoresis and unparalleled in its ability to solubilize protein;
preparation, no more also effective at inactivating proteases and other undesirable
than 0.2% (w/v)
enzymatic activities. It is, however, incompatible with IEF unless
during IEF
diluted to 0.2% or less and used with at least an eightfold excess of
an IEF-compatible detergent such as CHAPS
Dithiothreitol (DTT)
Reductant
20–60 mM
Most commonly used sulfhydryl reductant for 2-D electrophoresis
b-Mercaptoethanol
Reductant
1–5% (v/v)
Sulfhydryl reductant originally used for 2-D electrophoresis (O’Farrell
1975); must be used at a relatively high concentration and can cause
disturbances to IEF, so is rarely used
Tributylphosphine (TBP)
Reductant
2 mM
Phosphine reductant effective at low concentrations and reported
to enhance solubilization of recalcitrant samples (Herbert et al.
1998). It has low water solubility and is unstable and therefore not
recommended as the sole reductant for first-dimension IEF
Tris-carboxyethylphosphine Reductant
2–40 mM
Phosphine reductant that may be useful during sample preparation;
(TCEP)
it is highly charged and so is not recommended as the sole reductant
present during first-dimension IEF
Tris
Base
10–40 mM
(Unbuffered) free base often added to sample preparation solutions to
raise the pH to a range where proteolysis is minimal and proteins are
optimally soluble. Other bases (for example, potassium carbonate or
spermine) are occasionally used as well (Rabilloud 1999). If Tris is used
during sample preparation, it should be diluted to 20 mM or less for
first-dimension IEF, as it may cause disturbances in the basic pH range
Bio-Lyte
®
ampholytes
Carrier
0.2–1.0% (w/v)
Carrier ampholytes may be used during sample preparation to
ampholyte
enhance protein solubility. Although IEF with IPG strips does not
require carrier ampholytes for pH gradient generation, the presence
of a relatively low (0.2% [w/v]) concentration of carrier ampholyte
is essential for optimum resolution. Use pH 3–10 ampholytes
or ampholytes appropriate to the IPG strip pH range
18
19
2-D Electrophoresis Guide
Theory and Product Selection
Chapter 2: Sample Preparation
Removal of Interfering Substances
Impurities such as ionic detergents, lipids, nucleic
acids, salts and other ionic compounds, and
even high-abundance proteins can impact a 2-D
electrophoresis experiment by interfering with protein
separation or by obscuring proteins of interest.
These interfering substances can be endogenous
(for example, phenolics, lipids, and nucleic acids) or
exogenous (added during sample preparation; for
example, salts and detergents). Either way, removing
these impurities prior to analysis or mitigating their
effect is often essential for good results.
Though removal or mitigation of interfering
substances often yields clearer 2-D patterns and
improves resolution of protein spots, any treatment
of the sample can reduce yield and alter the relative
abundance of sample proteins. Procedures for
the removal of interfering substances represent
a compromise between removal of non-protein
contaminants and minimal interference with the
integrity and relative abundance of the sample
proteins. Since proteomics aims to study the
relationship among proteins in their natural state, it
is important to remove an interfering substance only
when necessary and by using techniques appropriate
for the sample.
Protein precipitation is a common general method
for contaminant removal. Conditions are chosen
under which sample proteins are selectively
precipitated while leaving soluble the major non-
protein contaminants. Following centrifugation, the
precipitated proteins are resuspended in a solution
suitable for IEF. Methods used in sample preparation
for 2-D electrophoresis include precipitation with
TCA and acetone (Damerval et al. 1986, Görg et al.
1988) and precipitation with methanol and chloroform
(Wessel and Flügge 1984). Precipitation procedures
also have the benefit of concentrating sample
protein, which is often necessary for effective
sample application.
Individual types of interfering contaminants cause
specific problems and can be removed or mitigated
in different ways. The most prevalent interfering
contaminants and their removal methods are
discussed next.
Nucleic Acids (DNA and RNA)
Nucleic acids, particularly DNA, can interfere with
IEF (for example by clogging gel pores) and increase
sample viscosity, thus limiting the effectiveness of cell
lysis and sample application. Because smaller nucleic
acids are generally tolerated better, strategies to
reduce nucleic acid interference involve either
shearing or enzymatic digestion: sonication shears
DNA and renders the sample less viscous, and
addition of nuclease digests nucleic acids to
oligo- or mononucleotides.
Nucleases are often employed during sample
preparation, particularly with bacterial lysates in
which nucleic acid:protein ratios are high. Successful
application of nuclease treatment requires attention
to three factors:
■
■
Nucleases may be inactive under the strongly
denaturing conditions often used to prepare protein
samples for 2-D electrophoresis
■
■
DNase requires magnesium ions for activity
■
■
Nucleases are proteins and can appear in the
2-D pattern as extra spots
Benzonase is a nuclease with properties that make
it particularly useful in sample preparation for 2-D
electrophoresis (Chan et al. 2002). It is active in
the presence of urea, and the amount required for
treatment is usually not visible in a 2-D gel. It is
applied in the presence of 1 mM MgSO
4
or MgCl
2
.
The magnesium ions are subsequently sequestered
with EDTA in order to inhibit proteases that may require
metal ions for activity.
