Reducing agents, Ampholytes, buffers, and other additives, Readyprep reduction-alkylation kit – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 10
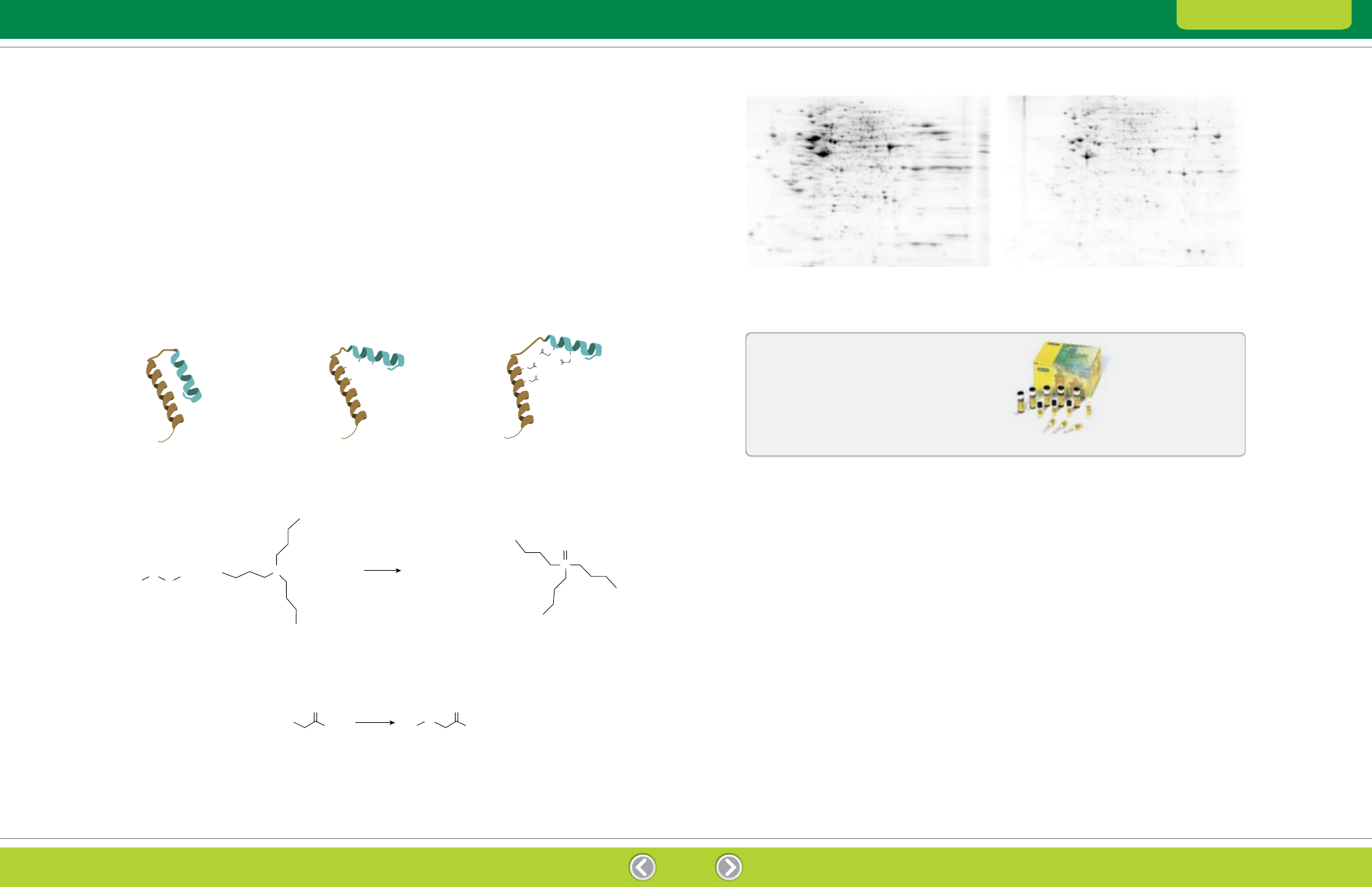
Fig. 2.1. Reduction and alkylation.
ReadyPrep Reduction-Alkylation Kit
Bio-Rad’s ReadyPrep reduction-alkylation kit
provides the reagents for reduction and alkylation of
sample proteins prior to IEF. Its use produces a 2-D
pattern with more spots, fewer streaks, and greater
reproducibility.
ReadyPrep Reduction-Alkylation Kit
16
17
2-D Electrophoresis Guide
Theory and Product Selection
Chapter 2: Sample Preparation
Reducing Agents
Reducing agents cleave disulfide bond cross-
links within and between protein subunits, thereby
promoting protein unfolding and maintaining proteins
in their fully reduced states. The compounds used
for 2-D sample preparation are either sulfhydryl or
phosphine reducing agents. Examples of sulfhydryl
reductants include dithiothreitol (DTT), dithioerythritol
(DTE), and
b-mercaptoethanol (BME). DTT and
DTE can be used at lower concentrations than
b-mercaptoethanol and are more commonly used, but
high concentrations of DTT can affect the pH gradient
since its pKa is around 8. Examples of phosphine
reductants include tributylphosphine (TBP) and Tris-
carboxyethylphosphine (TCEP). These reducing agents
can be used at lower concentrations and over a wider
pH range than the sulfhydryl reductants; however,
their use is limited by low solubility and instability (TBP)
or a highly charged characteristic (TCEP).
Reducing agents added during protein extraction help
to solubilize proteins; during IEF, however, reducing
agents such as DTT become depleted from the basic
end of pH gradients extending above pH 8, which can
cause proteins to aggregate and precipitate (Hoving
et al. 2002). The result is streaking and other random
spot patterns, particularly in the alkaline regions of
the IPG strip (Herbert et al. 2001). To address this
problem, proteins can be reduced with TBP and then
irreversibly alkylated with iodoacetamide (Figure 2.1).
This treatment blocks protein sulfhydryls and prevents
proteins from aggregating and precipitating due to
oxidative cross-linking, ensuring that proteins remain
soluble throughout electrophoresis (Figure 2.2).
Fig. 2.1. Reduction Alkylation
Disulfide
Tributylphosphine
Thiols
Reduction
Tributylphosphine oxide
H
2
O
O
R
1
S
P
S
R
2
R
1
—SH
+
R
2
—HS
+
+
+
Protein with disulfide bridges
Reduction cleaves disulfide bridges
and allows unfolding
Alkylation with iodoacetamide prevents
disulfide bridges from reforming
–S
–S
–
–S
–S
–
SH
SH HS
HS
O
NH
2
S
O
NH
2
S
O
H
2
N
S
O
H
2
N
S
P
Thiol
Iodoacetamide
Alkylated thiol
Alkylation
O
NH
2
R
S
R—SH
+
+
HI
I
O
NH
2
ReadyPrep reduction-alkylation kit
pH 3
pH 10
Untreated
pH 3
pH 10
Fig. 2.2. Effect of treatment with the ReadyPrep reduction-alkylation kit. Human HeLa cell extract (100 µg) separated by 2-D
electrophoresis (first dimension on 11 cm ReadyStrip
™
IPG strips pH 3–10, second dimension using 12% Criterion
™
gels) and stained with
Flamingo
™
protein gel stain. The sample treated with the ReadyPrep reduction alkylation kit (right) and shows much better spot resolution
than the untreated sample (left), especially in the basic range of the gel.
Ampholytes, Buffers, and Other Additives
Sample solution components that modify pH or
impart ionic strength affect the solubilization of
proteins during sample preparation and strongly
influence 2-D electrophoresis.
Carrier ampholyte mixtures increase both buffering
power and ionic strength. Unlike non-ampholytic
ions, they do not interfere with IEF and can, in fact,
improve protein solubility by “salting in” proteins
that are otherwise insoluble under IEF conditions.
In addition, carrier ampholytes can diminish protein-
matrix interactions, which tend to occur at the basic
end of an IPG strip and lead to streaking caused
by precipitation (Righetti and Gianazza 1987).
Carrier ampholytes are routinely added to solutions
used during IEF with IPG strips and can be of value
during protein extraction as well.
Since proteins are often more soluble and proteases
are less active at higher pH, a base such as Tris may
be included in a lysis solution to elevate pH.
Many proteins also require ions in solution for optimum
solubility. Normally, this is achieved by adding salt
to the sample solution; however, adding salt prior to
IEF increases conductivity and consequently limits
the voltage at which IEF can be performed until the
salt is eventually removed from the system. Ions also
leave the IPG strip during IEF, causing any protein
requiring ions for solubility to precipitate. Proteins also
become less soluble as they approach their pI; they
may precipitate at their pI in a phenomenon known as
isoelectric precipitation or pI fallout.
