Selection of ipg strips, Choice of ph gradient, Choice of ipg strip length – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 20: Estimation of pl, Estimation of pi
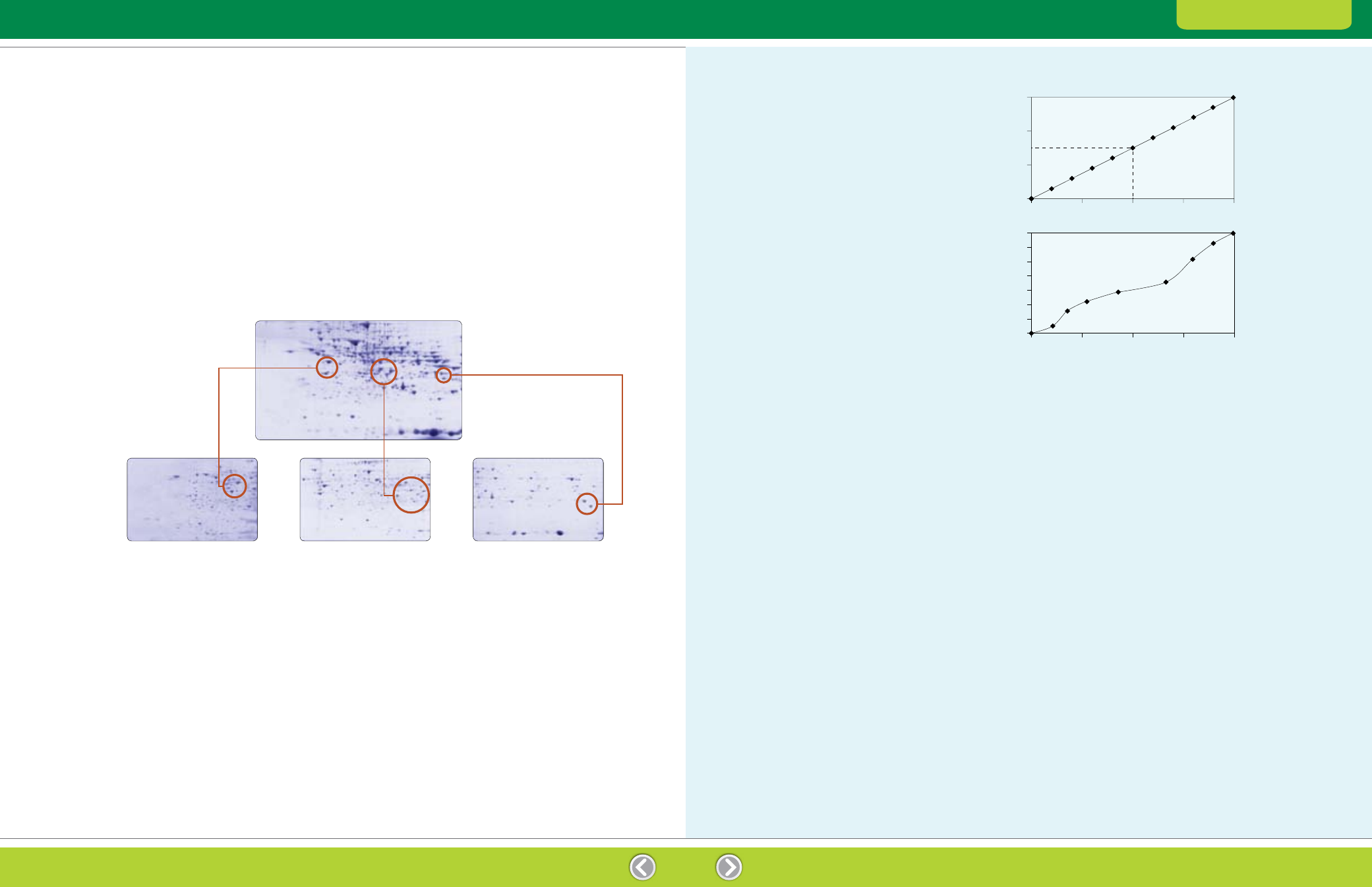
Fig. 3.3. A mouse liver sample was extracted in a urea-thiourea-CHAPS solution. The extract was run in a single PROTEAN
®
i12
™
IEF cell
run on twelve 11 cm ReadyStrip IPG strips simultaneously at each of the following pH ranges: 3–10, 3–6, 5–8, and 7–10. Each pH gradient was
run in triplicate. The second dimension for each IPG strip was run in 8–16% gradient Criterion
™
precast gels that were stained with Bio-Safe
™
Coomassie stain. The above figure shows a representative gel image for each pH range.
pH 3–10
pH 3–6
pH 5–8
pH 7–10
Estimation of pI
The pI of a protein is a useful parameter for protein
characterization. With purified proteins, pI can be
determined by IEF using IPG strips, usually under
denaturing conditions. Using linear IPG strips, the pH
gradient can be assumed to extend linearly between
the pH extremes. Knowing the length and pH range
of the IPG strip implies that experimental pI values can
be assigned with a high level of accuracy (see figure).
Protein pI estimations can also be made using NL
IPG strips, assuming the pH profile of the IPG strip is
available from the manufacturer; without the exact pH
profile of the strip, the pI estimate will be less accurate.
For pI estimation, stain the IPG strips after IEF,
for example with Bio-Safe Coomassie blue stain,
and then plot the migration distance along the length
of the IPG strips of each of the protein standards.
Graph A shows the pH gradient along the length
of a linear pH 4–7 IPG strip. To determine the pI of
an unknown, simply determine the band position
(as a percentage of gel length) and read the pI from
the graph. In the example, a band positioned at 50%
of the gel length will have an estimated pI of 5.5.
The same strategy can be applied for protein spots
on 2-D gels, but with less accuracy due to swelling
or shrinkage of the 2-D gel. It may also be difficult to
define the start and end positions of the IPG strip on
stained 2-D gels.
With knowledge of experimental pI and molecular
weight values (see Chapter 4 for details about
molecular weight estimation), it is possible to make
comparisons with the calculated values derived after
protein spot identification using mass spectrometry.
The calculation of theoretical pI values is possible with
software tools available on the Internet, for example
at http://web.expasy.org/compute_pi. If the values
differ significantly from each other, this may indicate
a false identification or the identification of a fragment
of the respective protein. However, differences in pI or
molecular weight can also suggest posttranslational
modifications, such as phosphorylation or glycosylation.
The detection of posttranslational modifications
is a unique strength of gel-based proteomics.
These modifications offer information about the
function, regulation, and cellular location of proteins.
Estimating the pI of a protein from its position along an IPG
strip. A, By plotting the pH of an IPG strip as a function of its
length, the pI of a protein may be derived from its focused position
on that strip. In the example shown, the pI of a protein that migrates
across 50% of the strip length is 5.5. B, pH profile of Bio-Rad
ReadyStrip nonlinear pH 3–10 IPG strips.
0
25
50
75
100
% Total IPG strip length
B. Nonlinear pH 3–10 ReadyStrip IPG strip
A. Linear pH 4–7 ReadyStrip IPG strip
0
25
50
75
100
pH
pH
10
9
8
7
6
5
4
3
7
6
5
4
36
37
2-D Electrophoresis Guide
Theory and Product Selection
Chapter 3: The First Dimension: Isoelectric Focusing (IEF)
Choice of IPG Strip Length
IPG strips are available in a variety of lengths that
match the size of most commercial second-dimension
vertical electrophoresis systems. Shorter strips match
mini-format systems, and longer strips match large-
format systems. Deciding which strips to use depends
on the requirements for speed, sample volume,
resolution, and throughput (see Chapter 4 for more
details on selecting size format for 2-D electrophoresis):
■
■
Use shorter strips and mini-format gels for
fast, convenient sample screening or method
development
■
■
Use longer strips for the best separation with
higher protein loads and for maximum resolution.
The longest IPG strips and large-format gels have
a large area to resolve protein spots; however, they
take a long time to run
Selection of IPG Strips
When selecting the IPG strip, consider both the pH
gradient and strip length, as both determine the
resolution in the final 2-D gel (see the ReadyStrip IPG
strips sidebar).
Choice of pH Gradient
IPG strips are available in various pH gradients
(see the table in the ReadyStrip IPG Strips sidebar).
The pH gradients are linear (pH varies in a linear
manner with respect to length of the strip) except in
the case of nonlinear pH 3–10 gradients (NL, see the
Estimation of pI sidebar).
■
■
Use broad-range strips (for example, pH 3–10)
for an overview of the spot distribution along the
pH gradient and for comparing different sample
preparation strategies. Since many proteins focus
■
■
Combine different size formats for various benefits.
For example, use a mini-format system for rapid
optimization of sample preparation methods, then
switch to a large format for thorough assessment of
a complex sample and identification of proteins of
interest. In many cases, a mini system and narrow-
range IPG strips can then be used to focus in on
proteins of interest
■
■
Use overlapping narrow- and micro-range IPG strips
to increase the effective length of pI resolution.
When three narrow-range overlapping ReadyStrip
IPG strips (pH 3–6, 5–8, 7–10) are used with the
Criterion system, for example, the resolution in the
first dimension (11 cm strip, pH 3–10 NL) is increased
from 11 to 26 cm. When four micro-range strips
are used, the resolution in the first dimension is
expanded from 11 to 44 cm
in the middle of the pH range 3–10, using NL
gradients can improve resolution of proteins in the
middle of that range and compress the width of the
extreme pH ranges at the ends of the gradients
■
■
Use narrow- and micro-range gradients for greater
resolution (there is a larger separation distance, more
cm of gel, per pH unit). With the exclusion of proteins
outside the pH range of the strip, more total protein
mass can be loaded per strip to also allow detection
of more proteins
■
■
Use overlapping pH ranges to increase resolution by
expanding a small pH range across the entire width
of a gel (Figure 3.3). This also allows the creation
of composite gels by matching spots from the
overlapping regions using imaging software
