Polysaccharides, Phenolic compounds, Lipids – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 12: Salts and other small ionic compounds, Prevention of keratin contamination, Products for contaminant removal
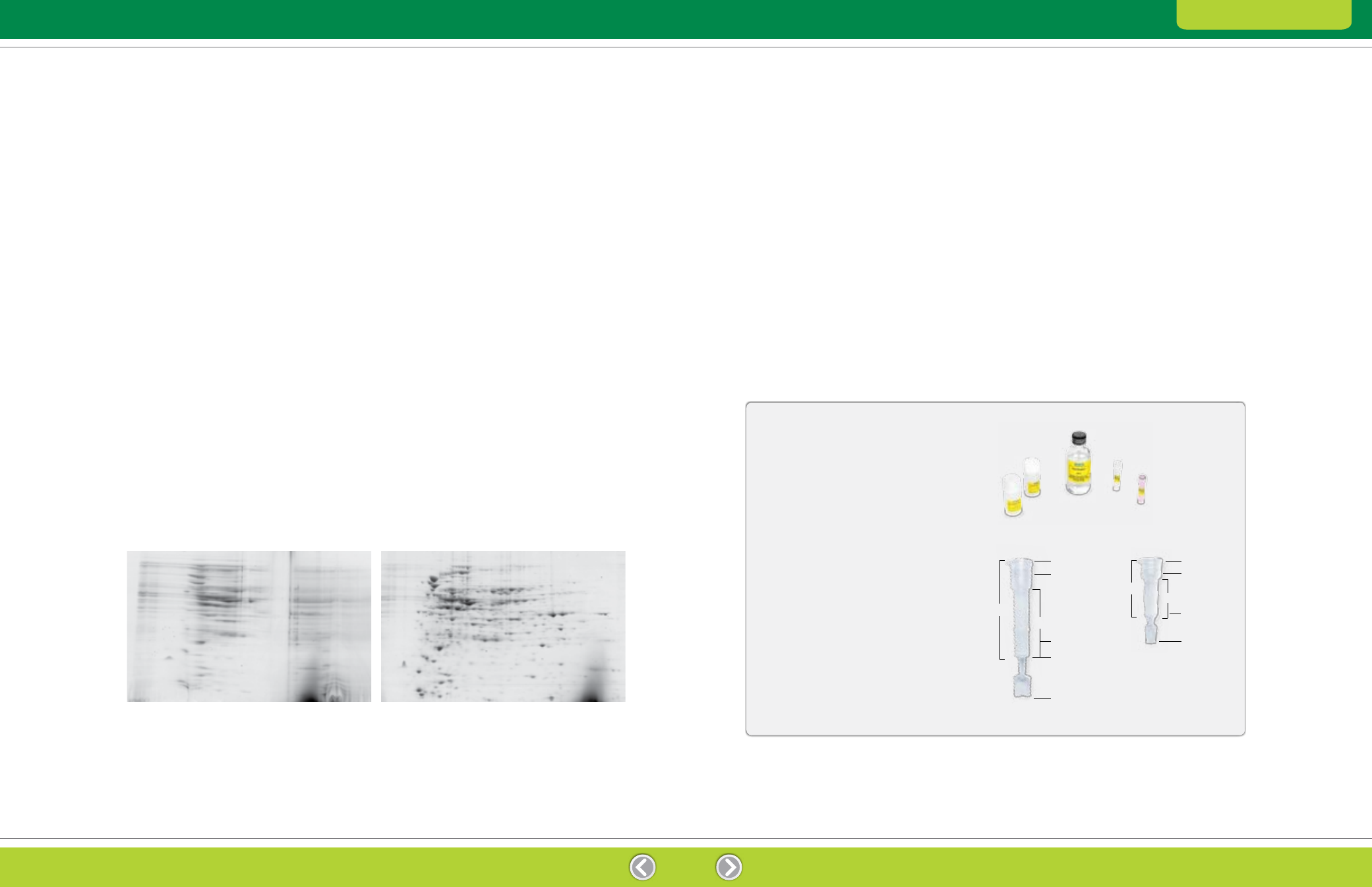
Before
After
Fig. 2.3. Effect of salt removal. E. coli extracts containing 1 M NaCl were separated by 2-D electrophoresis before and after treatment with
the ReadyPrep 2-D cleanup kit. The samples were focused using 11 cm ReadyStrip pH 3–10 IPG strips and then separated on Criterion 8–16%
Tris-HCl precast gels.
Products for Contaminant Removal
For quick and effective contaminant removal,
Bio-Rad offers:
■
■
ReadyPrep 2-D cleanup kit, which uses an
optimized version of a TCA-sodium deoxycholate
coprecipitation procedure (Arnold and Ulbrich-
Hoffmann 1999) to quantitatively precipitate
proteins while removing most interfering
substances. The protein precipitation process
also enables concentration of proteins from
samples that are too dilute, allowing for higher
protein loads that can improve spot detection
■
■
Bio-Spin
®
6 and Micro Bio-Spin
™
6 columns
are ready to use and are filled with Bio-Gel
®
P-6 support for the quick desalting and buffer
exchange of protein samples
ReadyPrep 2-D Cleanup Kit
2 cm working bed height
End cap
Reservoir
0.8 ml bed volume
Luer end fitting
with snap-off tip
3 cm
Micro Bio-Spin Column
5 cm
3.7 cm working bed height
End cap
Reservoir
Porous 30 µm
polyethylene bed
support retains
fine particles
Luer end fitting
with snap-off tip
1.2 ml bed volume
Bio-Spin Column
20
21
2-D Electrophoresis Guide
Theory and Product Selection
Chapter 2: Sample Preparation
Polysaccharides
Polysaccharides can interfere with electrophoresis by
clogging gel pores and by forming complexes with
proteins. Like nucleic acids, they can also cause a
sample to be viscous, making it difficult to work with.
Polysaccharides are a particularly prominent problem
with plant-derived samples.
Centrifugation may be used to remove high molecular
weight polysaccharides. Phenol extraction, followed
by precipitation with ammonium acetate in methanol,
is a commonly used method that is very effective
at removing polysaccharides in plant samples
(Hurkman and Tanaka 1986, Wang et al. 2008).
Phenolic Compounds
Phenolic compounds are found in all plants and
in some microorganisms and they can modify
proteins in an enzyme-catalyzed oxidative reaction.
The modification can cross-link proteins together or
render them insoluble. The reaction can be prevented
with reductants such as DTT,
b-mercaptoethanol,
or ascorbic acid, and the enzyme is inactivated
by thiourea. Phenolic compounds may also be
removed from the extract using the ReadyPrep
2-D cleanup kit (see the Products for Contaminant
Removal sidebar) or by including polyvinylpyrrolidone
(PVP) or polyvinylpolypyrrolidone (PVPP) in the
extraction solution. These compounds bind phenolic
compounds, and the precipitated complex can
be removed from the extract by centrifugation
(Toth and Pavia 2001). The phenol extraction
procedure described above (see Polysaccharides)
is also effective at removing phenolic contaminants
(Hurkman and Tanaka 1986, Wang et al. 2008).
Lipids
Lipids can form insoluble complexes with proteins,
but lipids can also complex with detergents, thereby
reducing the detergents’ effectiveness at solublilizing
protein. The effect of lipids can be minimized by
using excess detergent (for example, 4% CHAPS in
the lysis solution when preparing lipid-rich tissues
such as brain). Precipitation methods that employ
organic solvents (Damerval et al. 1986, Görg et al.
1988, Wessel and Flügge 1984) or the ReadyPrep 2-D
cleanup kit can also be used to remove lipids.
Salts and Other Small Ionic Compounds
IEF requires samples that are free of salts and other
small ionic compounds that may interfere with pH
gradient formation. Salts formed from strong acids
and strong bases (for example, NaCl) dissociate into
their component base and acid, which is eventually
drawn to either end of the IPG strip. Until this occurs,
the conductivity of the IPG strip remains high and the
voltage attained is low. The flow of ions from the IPG
strip is accompanied by water flow, and one end of
the strip may dry out, breaking electrical contact.
Weak acids and weak bases (for example, acetate,
Tris, or ammonium ions) may not completely leave the
IPG strip during focusing. These compounds interfere
with the pH gradient, resulting in streaking and loss
of resolution at one end of the pH range or the other
(Figure 2.3). Amphoteric buffers such as HEPES can
focus within the pH gradient, resulting in a portion of
the pH gradient where proteins focus poorly.
Samples of low ionic strength are desired, yet many
samples contain salts and small ionic compounds that
are either intrinsic to the sample type or have been
introduced during sample preparation. Precipitation
and dialysis methods are very effective at removing
ionic contaminants, as is treatment with a desalting
column (Chan et al. 2002).
Prevention of Keratin Contamination
Skin keratin is a common contaminant of 2-D gels
and mass spectra. It may appear in silver-stained and
fluorescently stained 2-D gels as an artifact focusing
near pH 5 in the 50–70 kD region, or as an irregular
but distinctive vertical streaking parallel to the
SDS-PAGE direction of migration. The best remedy
for this keratin artifact is to avoid introducing it into the
sample in the first place. Filter all monomer solutions,
stock sample buffers, gel buffers, and electrode
buffers through nitrocellulose and store them in
sealed containers; then, clean the electrophoresis
cell thoroughly with detergent. Above all, careful
sample handling is important when sensitive detection
methods are used, and gloves should be worn while
handling samples, solution, or equipment.
