Protein separation by isoelectric point (pi), Ief media: ipg strips vs. carrier ampholytes, Readystrip ipg strips – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 19: Supplied commercially and ready to use, Prepared on a plastic backing to simplify handling
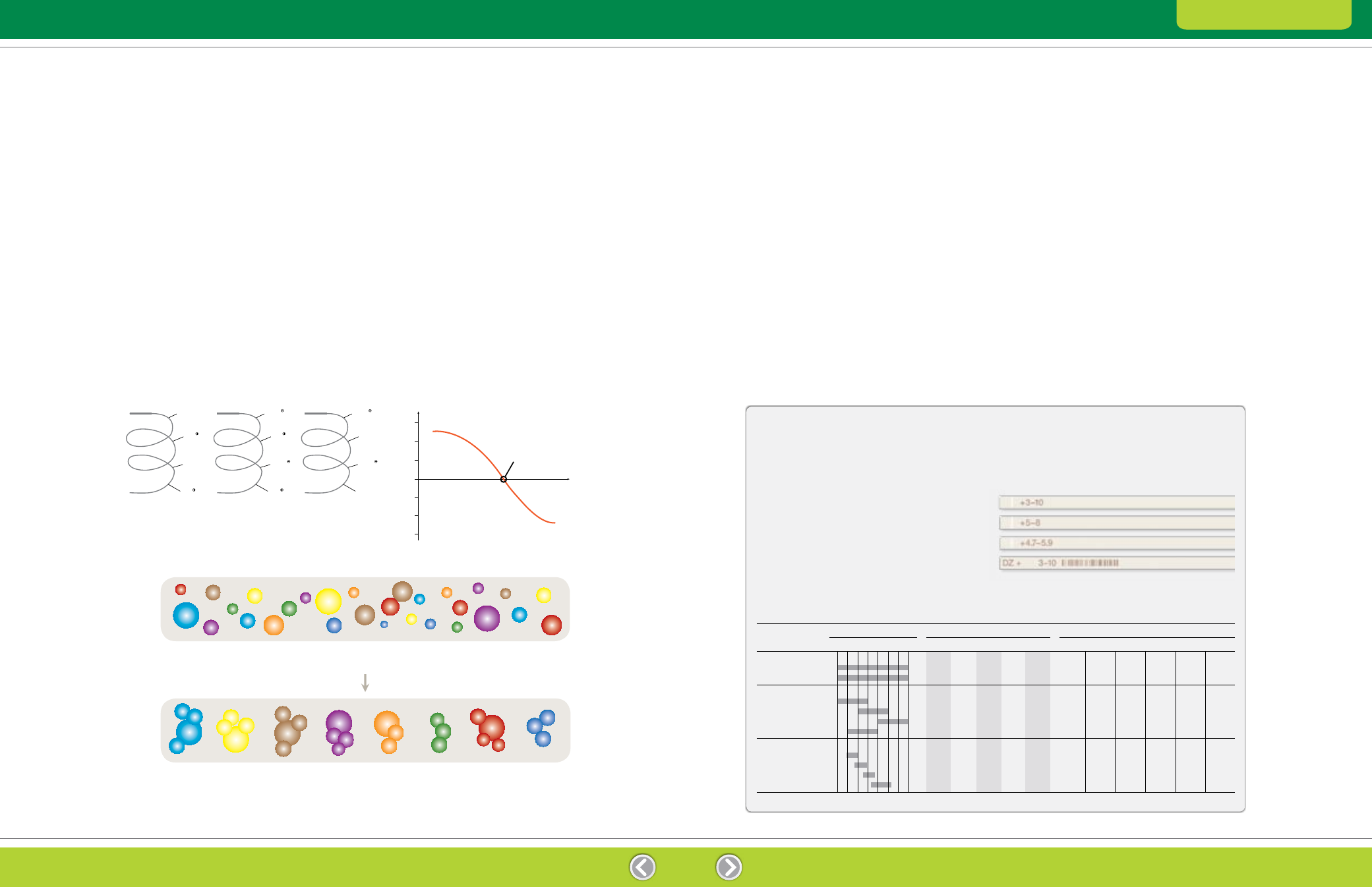
Fig. 3.1. Dependence of protein net charge on the pH of its environment. The pH at which the net charge is 0 is the isoelectric point (pI).
Net Charge
Isoelectric point (pl)
– 3
– 2
– 1
0
3
4
5
6
7
8
9
10
11 pH
pH < pl
COOH
COOH
+ 1
+ 2
+ 3
pH = pl
COO
COO
pH > pl
NH
2
NH
2
COO
COO
NH
3
NH
3
NH
3
NH
3
Net Charge
Isoelectric point (pl)
– 3
– 2
– 1
0
3
4
5
6
7
8
9
10
11 pH
pH < pl
COOH
COOH
+ 1
+ 2
+ 3
pH = pl
COO
COO
pH > pl
NH
2
NH
2
COO
COO
NH
3
NH
3
NH
3
NH
3
Fig. 3.2. Principle of IEF. A mixture of proteins is separated in a pH gradient and within an electric field according to each protein’s pI
and independently of its size. The proteins migrate until they reach their pI.
Anode
+
Cathode
–
pH
Anode
+
Cathode
–
pH
3 4 5 6 7 8 9 10
3 4 5 6 7 8 9 10
Focusing
3
4
6
8
8
8
8
9
10
8
9
3
3
4
4
4
4
3
3
3
4
5
5
5
5
6
6 6
9
6
9
9
7
7
7
9
9
10
10
10
7
7
3
4
5
6
3
4
5
8
10
6
6
7
5
5
9
10
ReadyStrip IPG Strips
IPG strips simplify first-dimension separations by
immobilizing the pH gradient on an easy-to-handle
support strip. ReadyStrip IPG strips are available
in a wide selection of pH gradients and strip
lengths (from 7 to 24 cm) to fit Bio-Rad vertical
electrophoresis cells and gels. Premade ReadyStrip
IEF buffers are also available for convenience and
maximum reproducibility.
Relative separation. Relative focusing power
expresses the enhanced resolution expected in the
first dimension when using IPG strips of different
lengths or pH ranges. The 7 cm pH 3–10 IPG strip
is arbitrarily assigned a baseline focusing power of
1.0 to calculate the relative focusing powers of the
other strips.
ReadyStrip IPG strips are preprinted to indicate anode (+) and pH range;
in addition, a bar code is printed on the 24 cm strip.
ReadyStrip IPG strip pH ranges.
pH
Relative Focusing Power
ReadyStrip IEF Buffer
Strip Range*
3 4 5 6 7 8 9 10
7
cm 11
cm 17
cm 18
cm 24
cm
3–10 7–10 3.9–5.1 4.7–5.9 5.5–6.7 6.3–8.3
Broad Range
3–10
1Ч
1.6Ч 2.4Ч 2.6Ч 3.4Ч
•
3–10 nonlinear (NL)
1Ч
1.6Ч 2.4Ч 2.6Ч 3.4Ч
•
Narrow range
3–6
2.3Ч 3.7Ч 5.7Ч 6.0Ч 8.0Ч
•
5–8
2.3Ч 3.7Ч 5.7Ч 6.0Ч 8.0Ч
•
7–10
2.3Ч 3.7Ч 5.7Ч 6.0Ч 8.0Ч
•
4–7
2.3Ч 3.7Ч 5.7Ч 6.0Ч 8.0Ч
•
Micro range
3.9–5.1
5.8Ч 9.2Ч 14.2Ч 15.0Ч 20.0Ч
•
4.7–5.9
5.8Ч 9.2Ч 14.2Ч 15.0Ч 20.0Ч
•
5.5–6.7
5.8Ч 9.2Ч 14.2Ч 15.0Ч 20.0Ч
•
6.3–8.3
3.5Ч 5.5Ч 8.5Ч 9.0Ч 12.0Ч
•
* Strips are designed with sufficient overlap to allow spot matching while limiting the extent of redundant data.
34
35
2-D Electrophoresis Guide
Theory and Product Selection
Chapter 3: The First Dimension: Isoelectric Focusing (IEF)
Protein Separation by Isoelectric point (pI)
The first-dimension separation of 2-D electrophoresis
is IEF, where proteins are separated on the basis of
differences in their pI. The pI of a protein is the pH at
which it carries no net charge, and it is a characteristic
that is determined by the number and types of
charged groups the protein carries.
Proteins are amphoteric molecules, which carry a
positive, negative, or zero net charge depending on
the pH of their environment. For every protein, there
is a specific pH at which its net charge is zero (its pI).
Proteins show considerable variation in pI, though pI
values usually fall in the range of pH 3–12, with the
majority falling between pH 4 and pH 8. A protein
is positively charged at pH values below its pI and
negatively charged at pH values above its pI (Figure 3.1).
For IEF, a protein is placed in a medium with a pH
gradient and subjected to an electric field. In response
to the field, the protein moves toward the electrode
with the opposite charge. Along the way, it either
picks up or loses protons. Its net charge and mobility
decrease until the protein eventually arrives at the point
in the pH gradient equal to its pI. There, the protein is
IEF Media: IPG Strips vs. Carrier Ampholytes
IEF for 2-D electrophoresis is most commonly
performed using immobilized pH gradient (IPG) strips.
As their name implies, IPG strips contain buffering
groups covalently bound to a polyacrylamide gel strip
to generate an immobilized pH gradient. The pH
gradients are created with sets of acrylamido buffers,
which are derivatives of acrylamide containing
both reactive double bonds and buffering groups.
The general structure is CH
2
=CH–CO–NH–R, where
R contains either a carboxyl [–COOH] or a tertiary
amino group (for example, –N(CH
3
)
2
). These acrylamide
derivatives are covalently incorporated into
polyacrylamide gels at the time of casting and
can form almost any pH gradient (Righetti 1990).
IPG strips are:
■
■
Supplied commercially and ready to use
■
■
Prepared on a plastic backing to simplify handling
■
■
Highly reproducible and stable over even extended
IEF runs (Bjellqvist et al. 1982)
■
■
Available in a wide variety of pH gradients and
lengths (see the ReadyStrip
™
IPG Strips sidebar)
Historically, first-dimension IEF was performed
using carrier ampholyte–generated pH gradients
and tube gels. This type of first dimension has been
largely superseded by the use of IPG strips for the
following reasons:
■
■
Carrier ampholyte tube gels must be cast by the user
■
■
Carrier ampholyte–generated pH gradients drift
over time and are, therefore, not as reproducible
as immobilized pH gradients
■
■
Carrier ampholytes are complex chemical
mixtures, and batch-to-batch variations affect the
characteristics of the pH gradient
■
■
Narrow pH gradients and gradients encompassing
the extremes of the pH range (below pH 4 and above
pH 9) cannot be accommodated
■
■
Tube gels can be difficult to handle
uncharged and stops migrating (Figure 3.2).
If, by diffusion, it drifts away from the point in the
gradient corresponding to its pI, it acquires charge
and is pulled back. In this way, proteins condense,
or are focused, into sharp bands in the pH gradient
at their characteristic pI values.
IEF proceeds until a steady state is reached.
Proteins approach their pI values at different rates
but remain relatively fixed at those pH values for
extended periods. This is in contrast to conventional
electrophoresis (for example, polyacrylamide gel
electrophoresis, or PAGE), where proteins continue
to move through the medium until the electric field is
removed. Moreover, in IEF, proteins migrate to their
steady-state positions from anywhere in the system.
IEF for 2-D electrophoresis is performed under
denaturing conditions so that proteins are completely
disaggregated and all charged groups are exposed
to the bulk solution. Consequently, resolution is best
under denaturing conditions. Complete denaturation
and solubilization are required to minimize aggregation
and intermolecular interactions, thus ensuring that
each protein is present in only one configuration.
