Tips for ief, Ipg strip rehydration and sample loading, Performing ief – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 45: Followed by ief, Ief with gel-side up
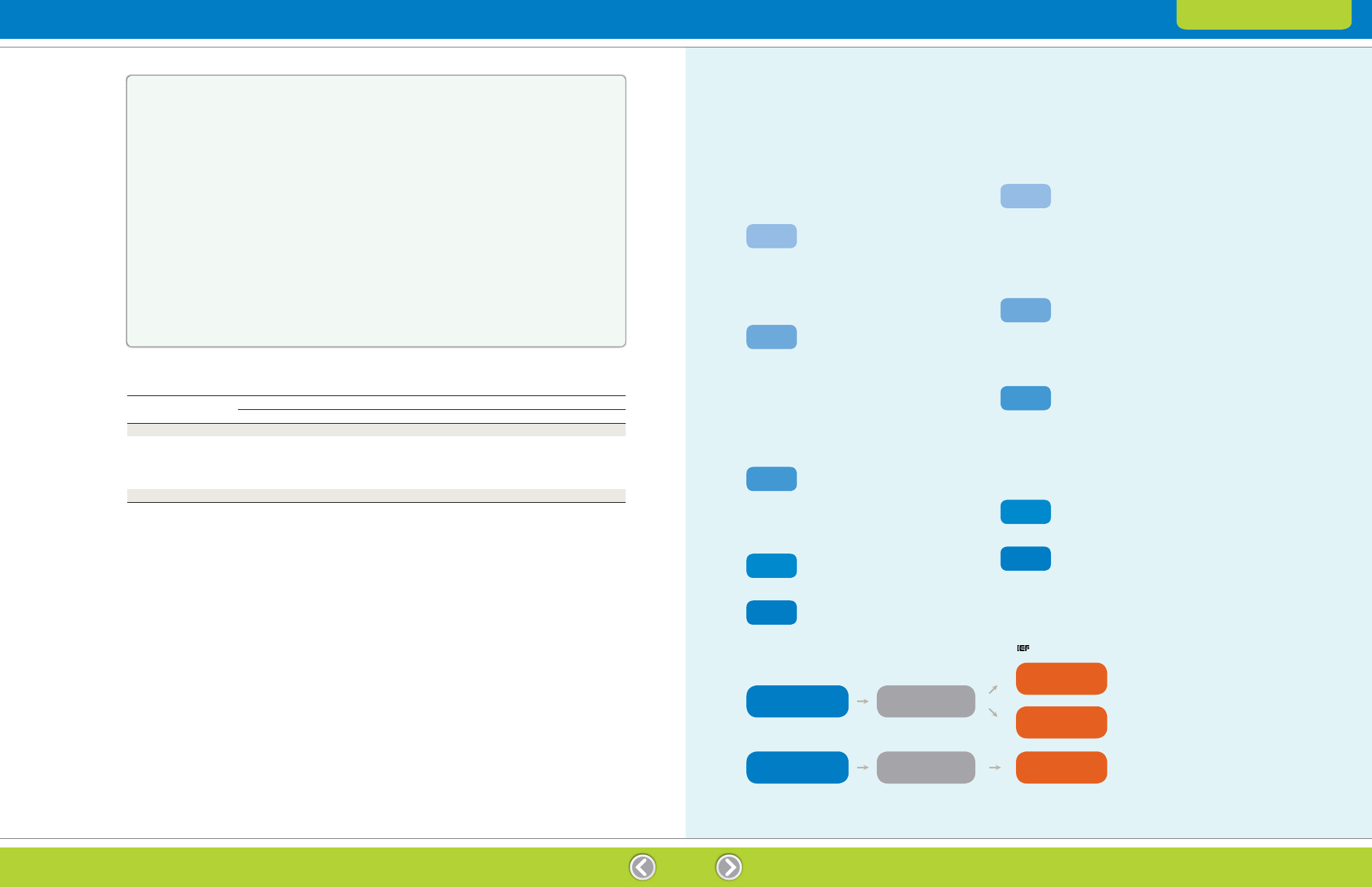
Table 9.1. Rehydration volumes and sample loads. Protein load recommendations are intended as a starting point, and the optimum amount
for the sample must be determined empirically. For narrow-range IPG strips, use more protein (proteins outside the range will not remain on the strip).
For single-pH-unit IPG strips, use up to 4–5 times more protein to improve the detection of low-abundance proteins.
IPG Strip Length, cm
7
11
17
18
24
Rehydration solution
125 µl
200 µl
300 µl
315 µl
450 µl
Protein
load
Coomassie (Brilliant) Blue
50–100 µg
100–200 µg
200–400 µg
200–400 µg
400–800 µg
Fluorescent stains
5–100 µg
20–200 µg
50–400 µg
50–400 µg
80–800 µg
Silver stains
5–20 µg
20–50 µg
50–80 µg
50–80 µg
80–150 µg
Mineral oil
4 ml
5 ml
7 ml
7 ml
9 ml
Rehydration
IEF
With sample
(in-gel sample loading)
With gel-side up
Without sample
With gel-side down
With gel-side up
(cup loading)
Transfer IPG strips
to focusing tray
Transfer IPG strips
to focusing tray
Fig. 9.1. Sample loading.
86
87
2-D Electrophoresis Guide
Methods
Chapter 9: First-Dimension IEF with IPG Strips
IPG Strip Rehydration and Sample Loading
Prior to their use in IEF, IPG strips must be
rehydrated (with or without sample) to their original
thickness with rehydration solution (Table 9.1),
which is often the 2-D sample solution
(see Chapter 8).
Tips for Rehydration and Sample Loading
■
■
Rehydrate IPG strips for 12 hr–overnight at 20°C
(or room temperature)
■
■
After rehydration in a rehydration/equilibration tray,
rinse and blot the IPG gel strips to remove excess
rehydration solution before transferring to the
focusing tray; otherwise, urea may crystallize
on the surface of the IPG strips
■
■
Moisten electrode wicks with deionized water.
They should be moist, not wet
Tips for IEF
■
■
Master 2-D separation techniques using the
ReadyPrep
™
2-D starter kit (catalog #163-2105)
before using your own samples. The kit contains
premixed reagents, a standard sample, and a
detailed and optimized protocol, which allows
you to become familiar with the 2-D workflow and
techniques while validating the performance of
your 2-D system
■
■
When preparing solutions, use clean and dust-free
vessels to avoid keratin contamination
■
■
Use highly purified laboratory water
(conductivity <2 µS)
■
■
Use deionized urea prepared with a mixed-bed ion
exchange resin to avoid protein carbamylation by
cyanate, which forms in old urea
■
■
Do not heat urea-containing buffers to >37°C to
avoid protein carbamylation
Pipet the rehydration solution
(with or without sample, see Table 9.1
for volumes and protein loads) along
the center of the channel(s) of the
i12 rehydration/equilibration tray.
Take care not to introduce air
bubbles when expelling the solution.
Using forceps, remove the cover sheet
from the IPG strip, then gently place
the IPG strip gel-side down onto the
solution in the channel. Move the IPG
strip back and forth slightly to ensure
that the solution is distributed along its
length and that the strip is not sticking
to the bottom of the tray. Take care to
avoid trapping air bubbles beneath the
IPG strip.
Overlay each IPG strip with mineral oil
to prevent evaporation and precipitation
of urea during rehydration (see Table 9.1
for recommended volumes). Apply the
mineral oil to both ends of the channel
and allow it to flow toward the middle.
Cover the tray and leave it on a
level bench overnight (12–18 hr) for
complete rehydration.
Transfer the rehydrated IPG strips to
the focusing tray for IEF (see below).
Using forceps, remove the IPG strips
from the rehydration tray, remove
excess mineral oil, and place the
rehydrated IPG strips gel-side up
in the channels of the focusing tray.
Position the positive (+) ends of the
IPG strips against the positioning
stops in each channel.
Recommended: Wet the gel-side
up wicks (notched) with distilled or
deionized water and blot off excess
water. Use two wicks per IPG strip:
place a wick at each end of each
IPG strip.
Position the electrode assemblies
in the focusing tray and press
down on the green tabs to snap
the electrode assemblies into place.
Place the focusing tray with the
rehydrated IPG strips on the Peltier
platform and connect the electrodes
to the instrument.
Overlay each IPG strip with
mineral oil (see Table 9.1 for
recommended volumes).
Select or program the protocol(s)
and start the run.
IPG Strip Rehydration in Rehydration/
Equilibration Trays Followed by IEF
The instructions in this chapter pertain to the use of
the PROTEAN
®
i12
™
cell and accessories. For more
details about the components of this system and their
assembly and use, please refer to the PROTEAN i12
cell instruction manual (bulletin 10022069).
Protocol
IEF with Gel-Side Up
The following protocol is for IPG strips that have
been rehydrated in the presence of sample
(in-gel sample loading).
Protocol
Performing IEF
1
1
2
3
4
5
2
3
4
5
