The context of proteomics, Overview of experimental design, D electrophoresis workflow – Bio-Rad GS-900™ Calibrated Densitometer User Manual
Page 5
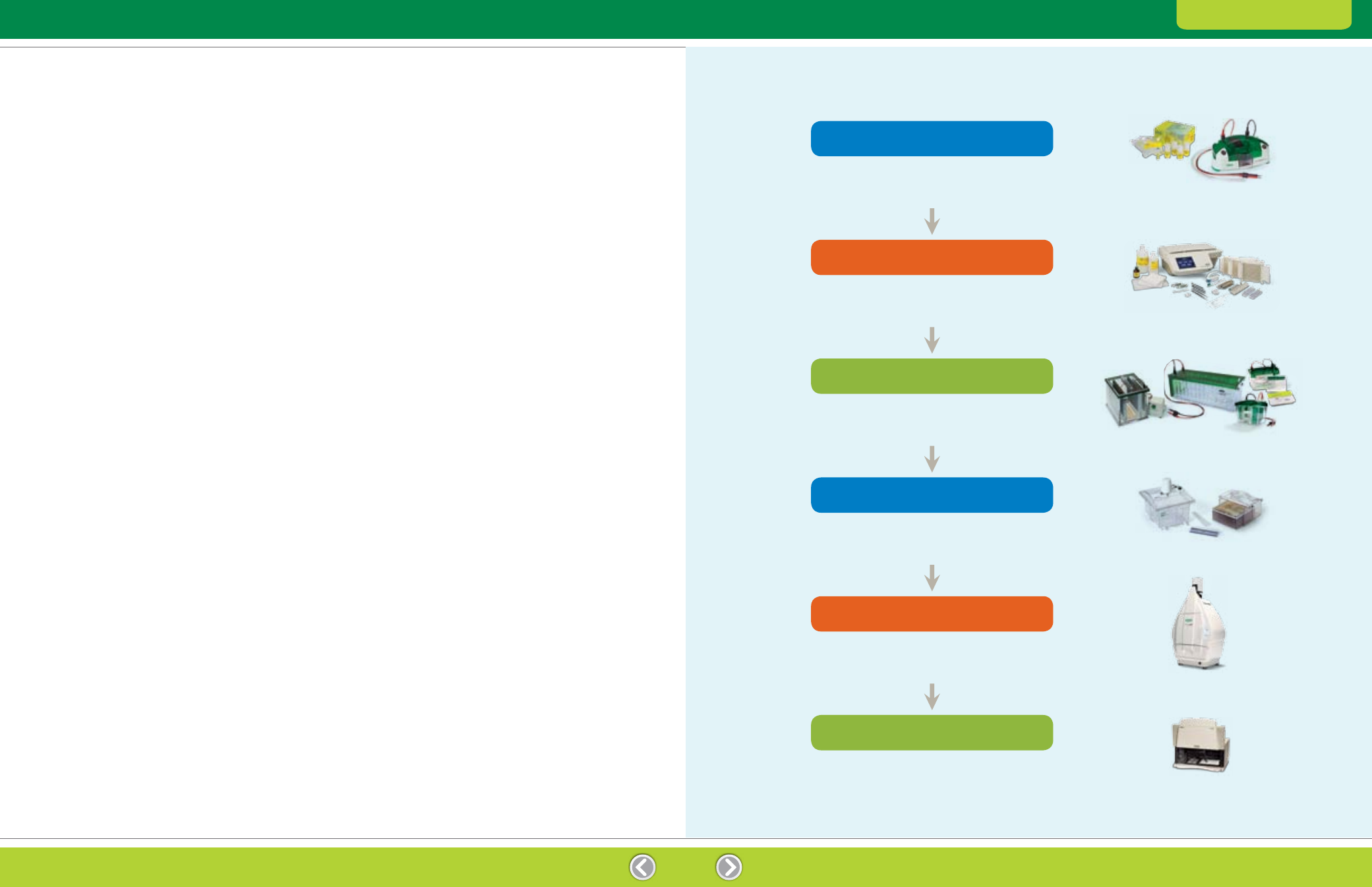
Fig. 1.1. General workflow for a 2-D experiment.
6
7
Theory and Product Selection
Chapter 1: Overview of Two-Dimensional Electrophoresis
Other common methods of proteome analysis involve
the proteolytic digestion of sample proteins and the
chromatographic separation of the resulting peptides
coupled directly to mass spectrometric analysis.
Peptides are identified by referencing a database,
and their proteins of origin are inferred. While these
methods are largely automatable and provide an
impressive depth of proteome coverage, some
information is lost when analyzing protein fragments
instead of intact proteins. The 2-D electrophoresis
approach maintains proteins in their intact states and
enables the study of isoform distribution, which is not
possible if the sample is proteolytically digested prior
to separation. Since proteins can be selected through
image analysis, mass spectrometry need be applied
only to the proteins of interest. This is an important
consideration when access to instrumentation or the
expense of mass spectrometric analysis is a limitation.
The suitability of 2-D electrophoresis to proteome
analysis is clear, but its applications also extend to
biomarker detection, development of drug and other
therapies, and optimization and development of
protein purification strategies.
Overview of Experimental Design
The general workflow in a 2-D electrophoresis
experiment (Figure 1.1) and some of the factors
affecting the way the experiment is performed
are outlined next.
The Context of Proteomics
Proteome analysis (proteomics) is the comprehensive
analysis of proteins present in a sample and
representing a particular physiological state at a
particular point in time. The aim of proteomics is to
determine the presence, relative abundance, and
posttranslational modification state of a large fraction
of the proteins in a sample (Wilkins et al. 1996).
Since proteins are directly involved in cellular structure,
regulation, and metabolism, proteomics can often yield
a more informative and accurate picture of the state of
a living cell than can analysis of the genome or mRNA.
One of the greatest challenges of proteome analysis
is the reproducible separation of complex protein
mixtures while retaining both qualitative and
quantitative relationships. Many combinations of
techniques can be used to separate and analyze
proteins, but two-dimensional (2-D) electrophoresis
is uniquely powerful in its ability to separate hundreds
to thousands of products simultaneously (Choe and
Lee 2000). This technique uses two different
electrophoretic separations, isoelectric focusing (IEF)
and SDS-PAGE, to separate proteins according to
their isoelectric point (pI) and molecular weight.
The identities of individual protein spots from the gel
can then be identified by mass spectrometry (MS)
of their tryptic peptides. Together with computer-
assisted image evaluation systems for comprehensive
qualitative and quantitative examination of proteomes,
proteome analysis also allows cataloguing and
comparison of data among groups of researchers.
Prepare the protein at a concentration and in a solution suitable
for IEF. Choose a method that maintains the native charge,
solubility, and relative abundance of proteins of interest.
Separate proteins according to pI by IEF. Select the appropriate IPG
strip length and pH gradient for the desired resolution and sample
load. Select appropriate sample loading and separation conditions.
Separate proteins according to size by SDS-PAGE.
Select the appropriate gel size and composition
and separation conditions.
Visualize proteins using either a total protein stain or fluorescent
protein tags. Select a staining technique that matches
sensitivity requirements and available imaging equipment.
Capture digital images of the 2-D protein patterns
using appropriate imaging equipment and software.
Then analyze the patterns using 2-D analysis software.
Excise protein spots of interest from the gel,
digest the proteins, and analyze the digests by MS.
Sample Preparation
First-Dimension Separation: IEF
Second-Dimension Separation: SDS-PAGE
Detection
Image Acquisition and Analysis
Protein Excision, Digestion, and Identification
2-D Electrophoresis Workflow
2-D Electrophoresis Guide
