Additional information, Accessories and calibration buffer solutions 55, 11 addi ti onal i nf orm at i on – Thermo Fisher Scientific CyberScan CON 10/CON 100/CON 200 User Manual
Page 56
53
11 ADDI TI ONAL I NF ORM AT I ON
What is Conductivity?
In general, conductivity is a value that represents how easily electrical charges can be
transported through a conductor. Conductors are substances that permit the movement of
electrical charge with relative ease.
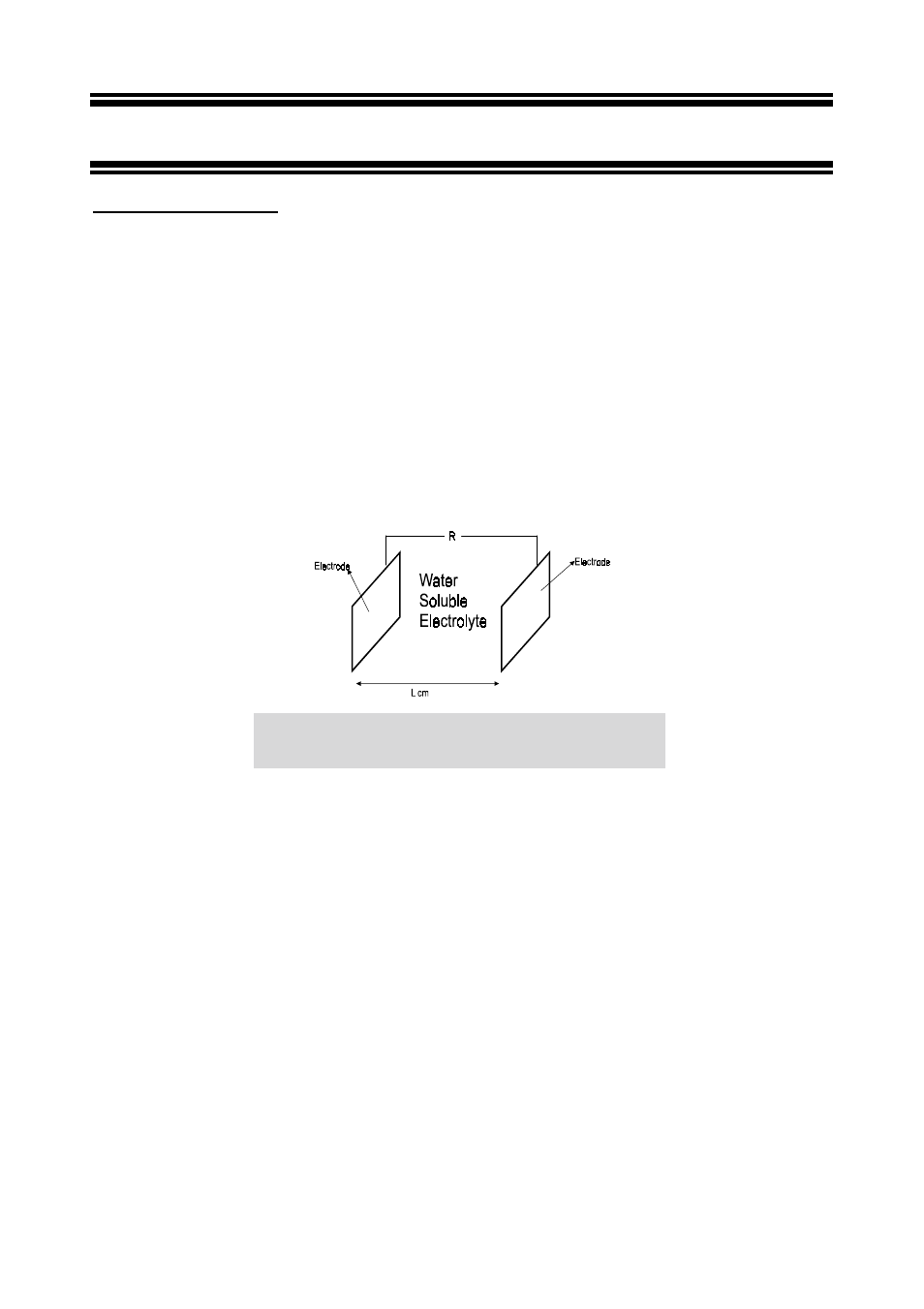
Figure 39 shows a conductivity cell. It is an electrochemical cell for measuring the conductivity
of an electrolyte solution. This cell consists of two electrodes; an anode and a cathode,
separated by an electrolyte solution. The two electrodes are in the shape of plates of identical
size, both having an identical surface area of A cm
2
. They are aligned parallel and are
separated by the distance, L. The space between them is filled completely with water-soluble
electrolyte solution. Alternating current flows through both electrode plates.
The negatively charged ions (anions) in the electrolyte migrate towards the anode and the
positively charged ions (cations) move towards the cathode. The result is the flow of electrical
current by ion movement.
The resistance to the movement of the charge between the two electrodes is inversely
proportional to their surface area and in direct proportion to their surface and also in direct
proportion to the distance between them. This is true for both electron movements in the metal
electrodes and ion movement in electrolytes. This relationship may be expressed by the
equation below.
R = r. (L/A) = r.k Equation 1
Figure 54: Conductivity cell. An electrochemical cell for
measuring conductivity.
