Sample volume, Sample dilution, Sample volume sample dilution – Luminex xPONENT 3.1 Rev 2 (IVD) User Manual
Page 15
set. Since each bead is analyzed individually, even when the sets are mixed in a multiplex
assay they can still be distinguished by their emission signals. The fluorescence signal of
reporter molecules bound to the surface of each bead set is measured and used to determine
the result of each assay in a multiplex. Again, since each bead is analyzed individually,
reporter signals for each bead set can be accurately quantified.
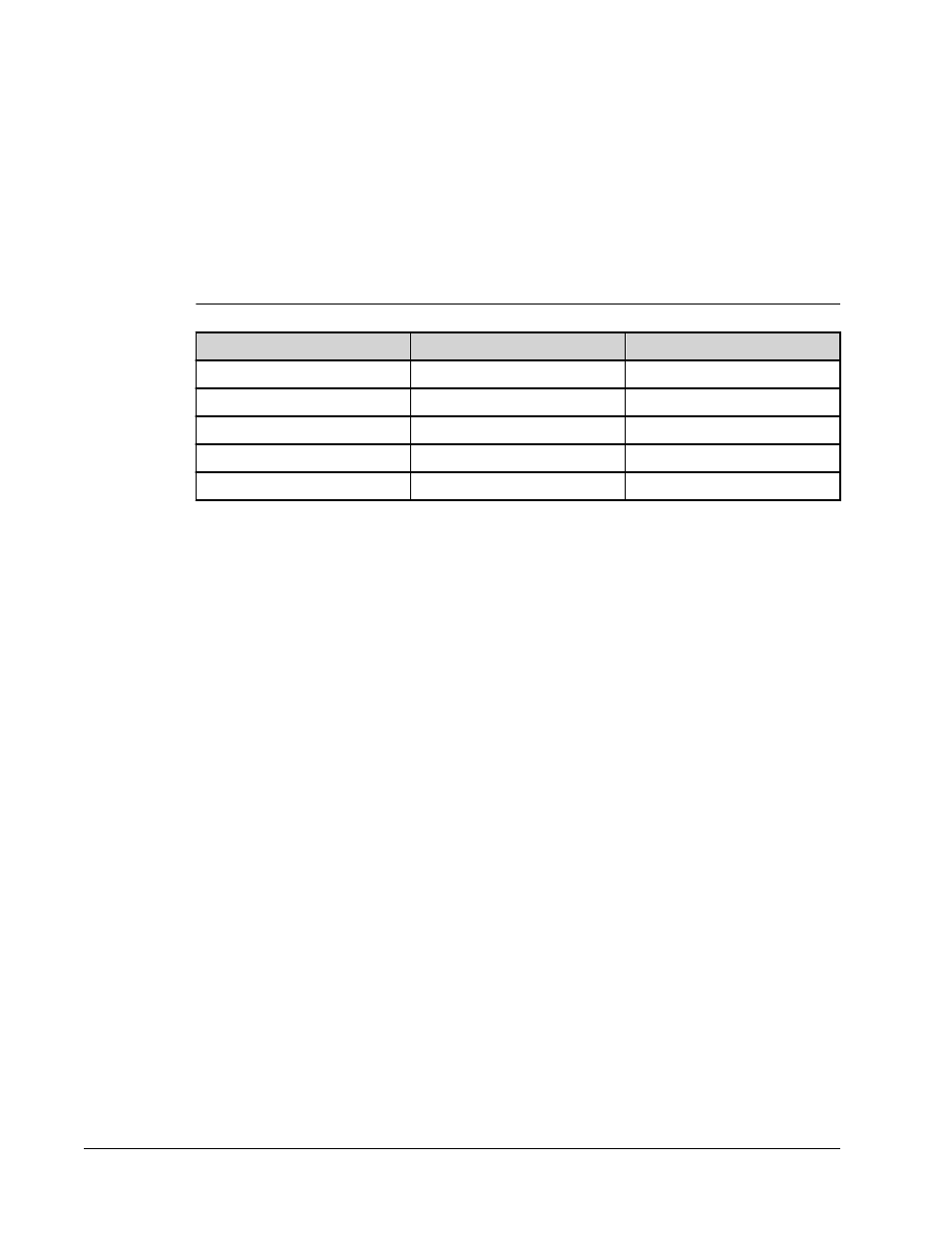
The following table displays acceptable reporter fluorochromes and their excitation and
emission wavelengths.
TABLE 3.
Reporter Fluorchromes Wavelengths
R-Phycoerythrin
Alexa 532
Formula weight (Daltons)
240,000
470
Absorbance max (nm)
480, 546, 565
531
Extinction max (M-1cm-1)
1,960,000
83,800
Emission max (nm)
578
554
Quantum yield
0.82
0.8
Sample Volume
Sample volumes or sample sizes range from 10 to 200 µL. Ensure that some sample remains
in the well after aspiration; about 25 µL greater than the sample volume. This amount may
vary depending on the type of plate used. After acquisition, the Luminex analyzer washes the
sample lines resulting in ejection of approximately 150165 µL of sheath fluid back into the
well for a 96-well plate. Ensure that there is room to add this amount to the well without
overflowing and contaminating other wells. Follow the IVD kit package insert instructions for
use.
The volume can be expounded by the following formula:
Total well volume (µL) – Sample uptake volume (µL) + 150165 (µL)
• Total well volume = Starting sample volume of a well before the unit samples for
acquisition. Well volume is determined by the consistency of the bead set.
• Sample uptake volume = Uptake volume for acquisition (program this in the protocol as
sample volume).
• 165 (µL) = Volume expelled back as stated in the above paragraph.
• Maximum well volume plate = The maximum volume capacity of the wells in a selected 96-
well microtiter plate.
Sample Dilution
Dilute concentrated biological samples such as plasma or serum following the IVD kit
package insert instructions for use. If running an xMAP-based kit, follow the dilution
instructions in the kit’s product insert.
For In Vitro Diagnostic Use
Introduction
5
