Titration theory – Hanna Instruments HI 903 User Manual
Page 209
17
TITRATION THEORY
3
INTRODUCTION TO TITRATION APPARATUS AND TYPICAL
TITRATION PROCEDURE
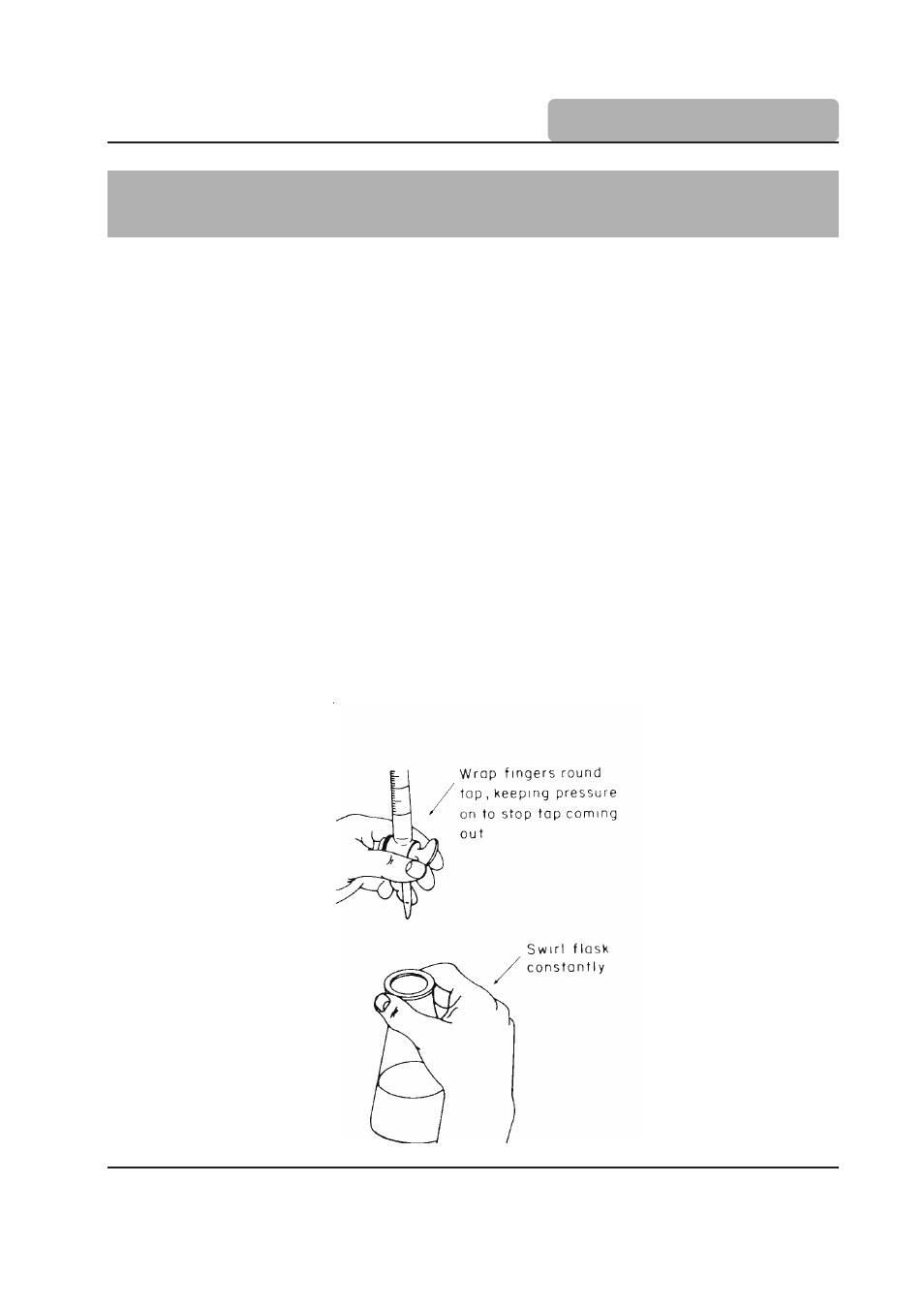
3.1 Manual Titration
Apparatus required for manual titration include:
• Volumetric Burette, for precisely controlled delivery of titrant to the reaction vessel;
• An Erlenmeyer, or similar flask, that facilitates constant mixing or swirling required to
ensure solution homogeneity;
• Volumetric pipettes for the precise addition of samples and indicator solutions;
• Standard titrant solutions of known concentration;
• A visual or instrumental indicator for detecting the completion of the reaction.
A typical manual titration consists of the following steps:
1. A volumetric pipette is typically used to add a known volume of sample to the flask;
2. An indicator solution or instrument probe is added to the flask;
3. A burette is used to measure the addition of titrant to the flask and dispense titrant in
a controlled manner;
4. Titrant is added via the burette until the method indication signals the reaction endpoint;
5. The concentration of analyte is calculated based on the concentration and volume of
titrant required to reach the endpoint and the reaction stoichiometry.
