Titration theory, 2 acid-base titrations – Hanna Instruments HI 903 User Manual
Page 203
11
TITRATION THEORY
Bivoltametric indication involves measuring the voltage required to maintain a constant current
flow between electrode elements. A small direct or alternating current called a polarization
current (Ipol) is applied between the electrode pins or rings and the resulting voltage is
measured in order to monitor the titration progress.
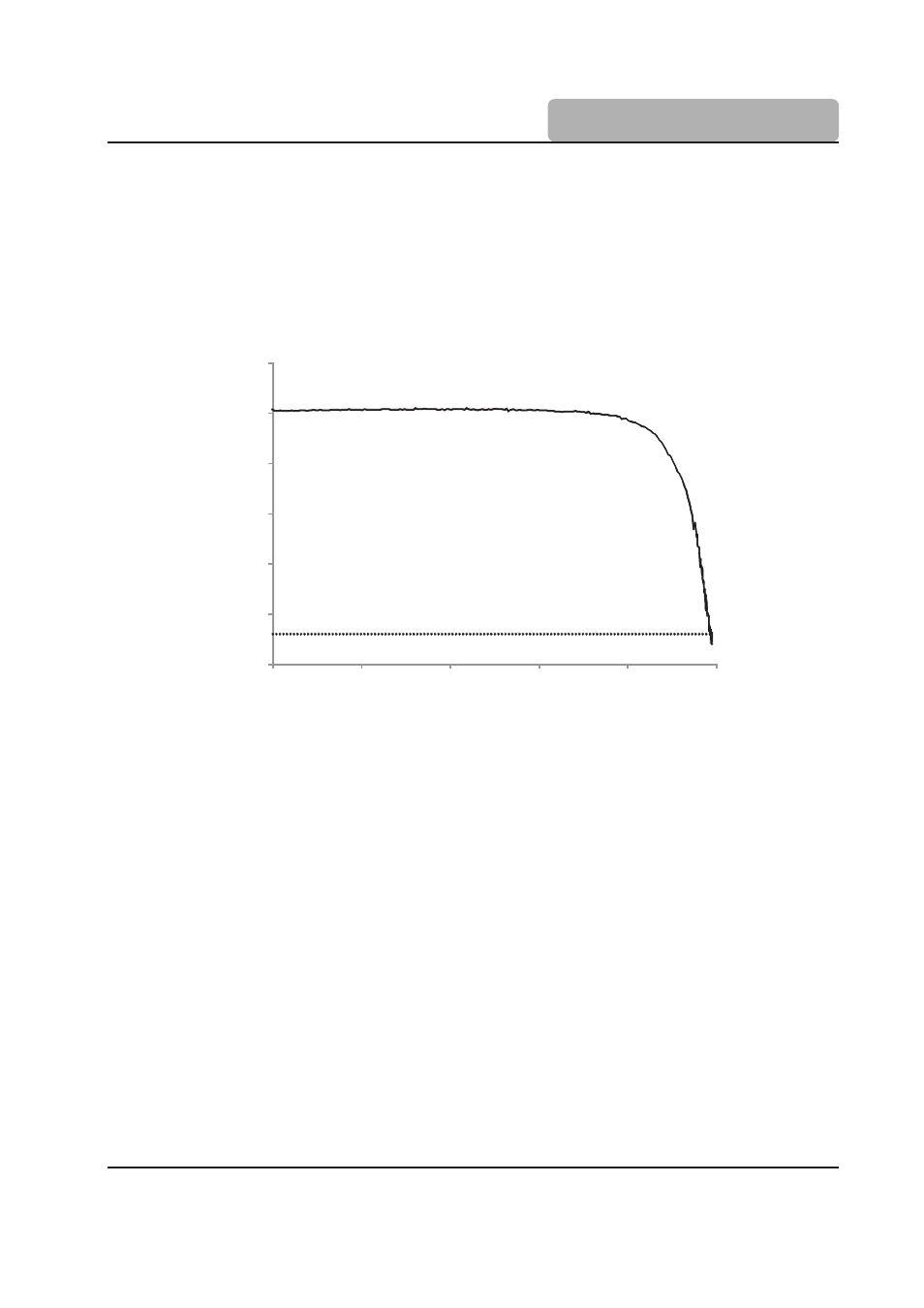
L-shaped titration curves are generated for both methods by plotting either the electrode
current or voltage against the volume of titrant added during the titration.
Electrometric methods result in over-titration or titration past the equivalence point where excess
iodine is present in the titration solution. Titration past the equivalence point is acceptable for two
reasons. First, due to the sensitivity of the electrometric methods, titrations are always carried
out to the exact same, slight excess of iodine resulting in highly reproducible titrations. Second,
the accuracy of electrometrically indicated titrations are not affected by the over-titration because
the slight excess of iodine has been accounted for during the standardization of the titrant.
2.2.2 Acid-Base Titrations
Acid–base titrations are the most common type of titrations. Acid–base titrations are based
upon a reaction between an acid and a base, a stoichiometric neutralization, or the exchange
of protons. Virtually all acid-base titrations are carried out using a strong acid or a strong
base as the titrant. The endpoint of a titration carried out with a weak acid or a weak base,
would be difficult to detect due to a small change in pH at the equivalence point.
Chemical indicators are often used to determine the endpoint. The indicator will change
color to signify that the end of the titration has been reached. When choosing the proper
indicator you should select one that has a pKa as close to the endpoint of the titration. The
color-change region of the indicator is usually ± 1 pH unit around the pKa. The theoretical
titration curve is useful for illustrating how the solution will change during the real titration,
and allowing the proper selection of an endpoint or an indicator.
150
200
250
300
350
400
450
0
0.2
0.4
0.6
0.8
1
Potential (mV)
Titrant Volume (mL)
Karl Fischer Titration, Bivoltametric Indication vs. Titrant Volume
