Titration theory – Hanna Instruments HI 903 User Manual
Page 199
7
TITRATION THEORY
2
TYPES OF TITRATIONS
2.1 Titrations According to The Measurement Method
2.1.1 Amperometric Titrations
An amperometric titration is performed by placing two electrodes (often a metal electrode and a
reference electrode) into the sample solution and holding the potential of the metal electrode at
a selected voltage. The current that flows, due to the oxidation or reduction of a reactant or
product, is plotted vs. volume of titrant to provide the titration curve and locate the equivalence
point. Changes in the current are due to changes in the concentration of a particular species
(being oxidized or reduced at the electrode).
Generally the reaction between the analyte and titrant forms a new species. Depending on the
titration, the reactants are electroactive and the products are not, or vice-versa. Amperometric
titration curves look like two straight lines intersecting at the equivalence point, this is due to the
change in the electroactivity of the solution.
Many metal ions can be amperometrically titrated using a precipitation, complexation or redox
reaction. Some metal ions and species that can be determined in this manner include silver,
barium, halides, potassium, magnesium, palladium, molybdate, sulfate, tungstate, zinc, bismuth,
cadmium, fluoride, indium, thallium, iodine, and gold.
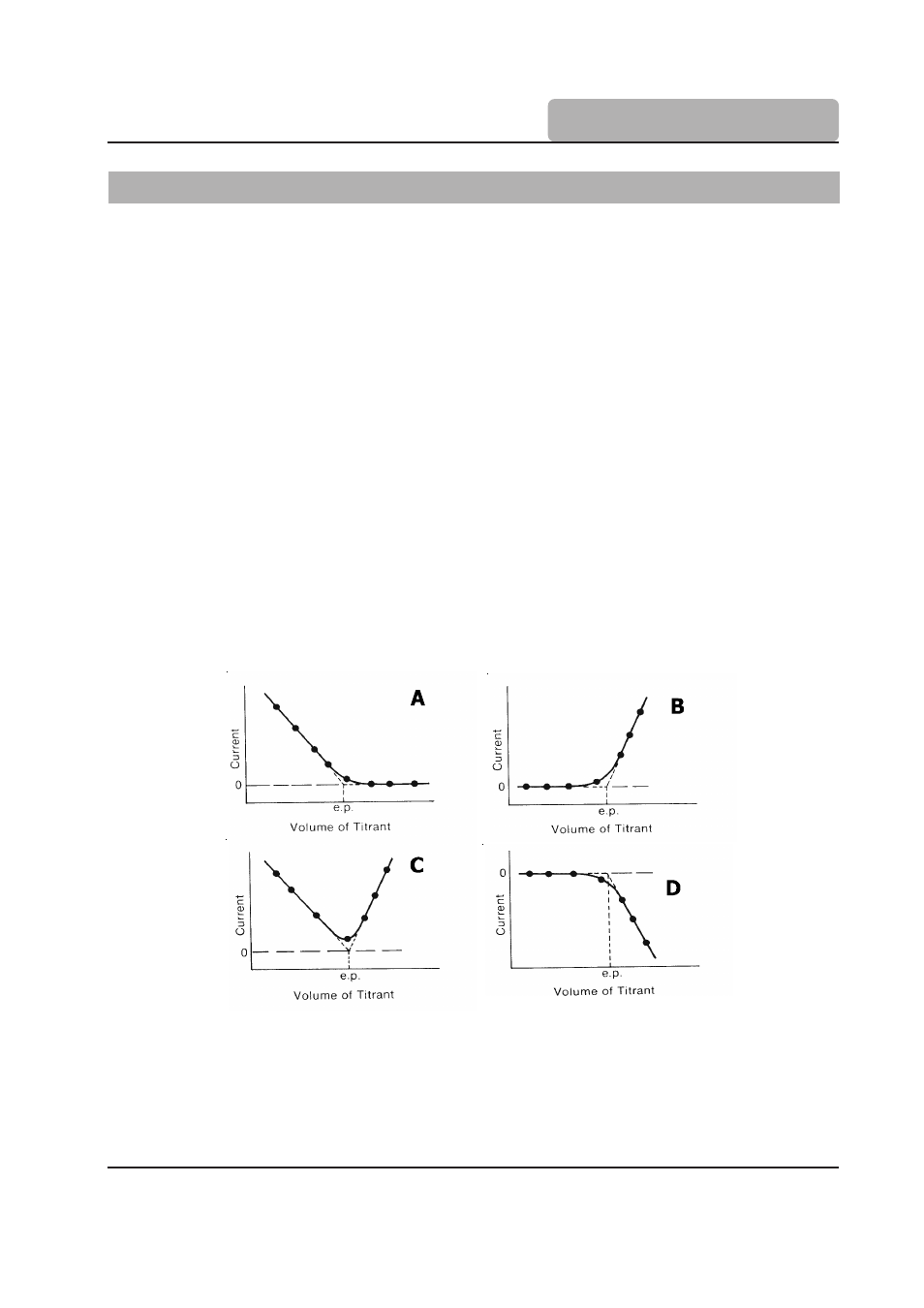
Figure 1 shows four amperometric titrations and their endpoints. In graph “A” the analyte is
electroactive and gives current but the reacted species does not. In “B” the reactant is not active
but the titrant is. In “C” both the analyte and titrant are active and both give current flow. Graph “D”
shows the same situation as “B”; however, the current has an opposite sign (the titrant is reduced).
2.1.2 Potentiometric Titrations
Potentiometric titrations are done by measuring the voltage across the solution using an electrode
system. An electrode system consists of an indicator electrode and a reference electrode. As
titrant is added the variations in the potential of the indicator electrode, with respect to the reference
Figure 1.
