Ph buffer use and maintenance – Metex 8760CLP Total Free Chlorine & pH Analyzer User Manual
Page 37
AQUAMETRIX INC.
1-800-742-1413 www.aquametrix.com
37
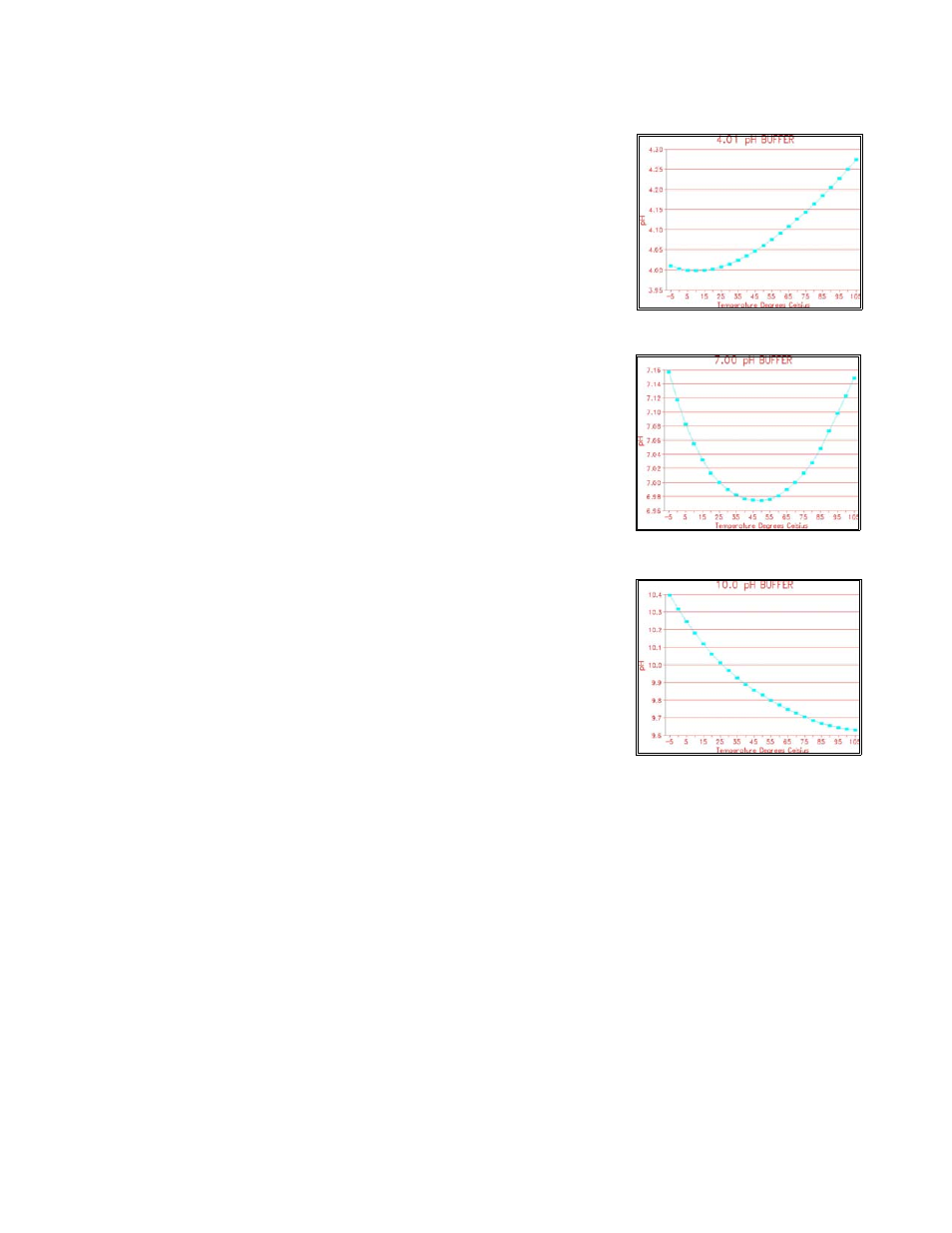
Temperature Dependence of Buffers
The pH of a solution is dependent on temperature.
To achieve greater accuracy, the temperature-
compensated values for the 4 pH, 7 pH and 10 pH
buffers are calculated by AquaMertix analyzers.
The graphs show the temperature-dependence of
the standard buffers. The TC-curves have been
programmed into the AquaMetrix analyzer. The
actual pH value of each of the three standard
buffers will be used.
Example: Calibrate using the pH 4.01 buffer (at
25 °C). The temperature of the buffer is 50 °C.
The analyzer will use the pH value of 4.05.
Incorrect Buffer Selection by the Analyzer
If the offset is known to be greater than ± 77 mV,
or if the analyzer selected the wrong buffer using
automatic buffer recognition, then it is necessary
to specify which buffer is being used. This is
done by selecting [4.01], [7.00], or [10.0] then an
offset of ± 4 pH units is allowed and
temperature-compensated values are still used.
Other Buffer Values or Custom Buffers
If a buffer with a pH value other than pH 4, pH 7,
or pH 10 is to be used, select [cuSt] (custom
value), then enter a value between 0 pH and
14 .pH. Buffer values entered this way are not
temperature compensated; the buffer is assumed
to have the specified pH value at the current
temperature. Offsets of up to ± 4 pH units are
allowed.
pH Buffer Use and Maintenance
A pH measurement is only as good as the calibration, and the calibration is only as good as the buffers.
The following guidelines for buffer maintenance will ensure accurate pH calibration and thus accurate
pH measurement.
•
Buffers have a limited shelf life. AquaMetrix suggests a one (1) year shelf life for unopened pH buffers. Store
buffers at room temperature.
•
Discard used buffer - do not return used buffer to the stock bottle.
•
Protect buffers from exposure to air as atmospheric carbon dioxide lowers the pH value of alkaline buffers.
Other trace gases found in industrial environments may also affect the buffer pH. Molds resulting from
airborne spores may accumulate in neutral and acidic buffers and change the pH value as well.
•
Rinse sensor with demineralized water before placing in buffer to prevent carryover contamination. A few
drops of demineralized water will not visibly alter the pH. Do not wipe the sensor dry as wiping may
induce a static charge which could result in noisy readings.
Illustration 33
: Temperature compensated pH 10 buffer
Illustration 32
: Temperature compensated pH 7 buffer
Illustration 31
: Temperature compensated pH 4 buffer
