Electrical equivalent of heat – PASCO PS-2006 GLX Power Amplifier User Manual
Page 23
®
P S - 2 0 0 6 G L X P o w e r A m p l i f i e r
S a m p l e E x p e r i m e n ts : E l e c t r i c a l E q u i v a l e n t o f H e a t
21
Electrical Equivalent of Heat
In this experiment, the Power Amplifier delivers a known quantity of
energy into the calorimeter, and the resulting temperature change is
measured.
Theory
The relationship between the heat (Q) added to a solid or liquid and the
resulting change in temperature (
∆T) is given by
(eq. 6)
Where c is specific heat and m is mass.
There are two things changing temperature in this experiment: the water
and the inner aluminum cup of the calorimeter. The water and cup have
different masses and different specific heats, but they both experience
the same temperature change.
Heat is added to the calorimeter by the use of a heating resistor. The
Power Amplifier supplies power to the heating resistor.
Electrical power is determined by the output voltage (V) and current (I):
(eq. 7)
Power is the rate of energy, thus energy is the area under a Power versus
time graph.
Pre-lab
1.
Measure the mass of the inner calorimeter cup.
2.
Add about 50 g of cool water to the inner cup. The water should ini-
tially be about 5° C below room temperature. After the cup and
water have come to equilibrium and you are ready to start the
experiment, it should be about 3° C below room temperature.
3.
Measure the mass of the cup plus water, and calculate the mass of
the water.
Setup
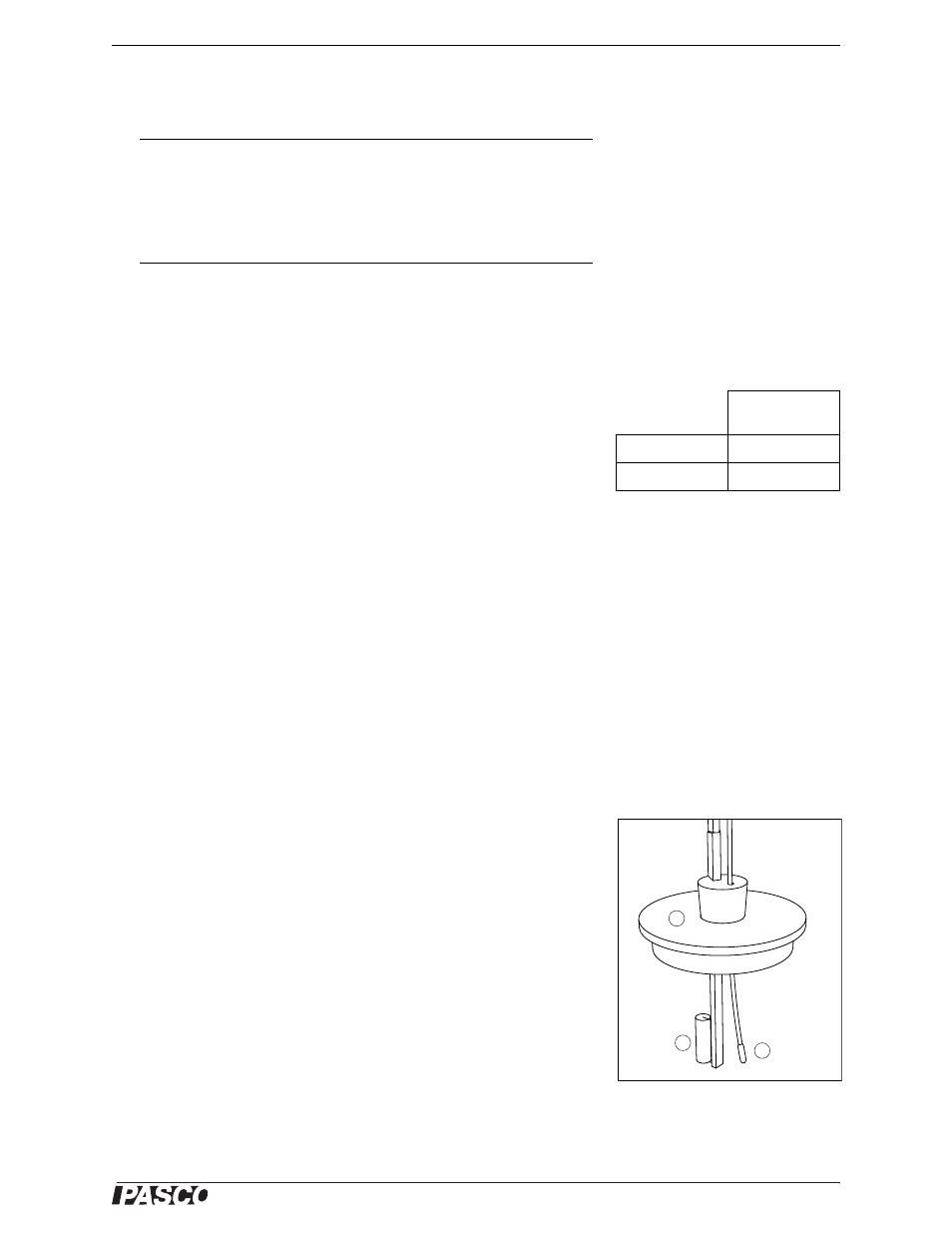
1.
Insert the fast response temperature probe into the calorimeter lid as
illustrated (Figure 20). The tip of the probe should be about 1 cm
from the heating resistor. Put the tip of a pencil into the rubber stop-
per to hold the temperature probe in place.
Additional Equipment
Part Number
Energy Transfer Calorimeter
ET-8499
Fast Response Temperature Probe PS-2135 (included with GLX)
Mass Balance
SE-8757A
Cool water (about 500 g)
specific heat
(J g
-1
K
-1
)
water
4.2
aluminum
0.9
Q
mc
∆T
=
P
IV
=
a
b
c
Figure 20: Equipment Setup
(a) calorimeter lid (b) heating resistor
(c) temperature probe
