ITC VerifyNow System Platelet Reactivity Test User Manual
Page 15
Page 11
14439.C 03/2013
Overview of System Components - 2
VerifyNow System User Manual |
USA
2.3
Sample Collection Tubes
The VerifyNow System uses whole blood collected in a partial-fill vacuum tube. During the test, the tube
is inserted onto the needle in the sample well of the test device - requiring no cap removal, specimen
preparation, or pipetting step. There are several types of sample collection tubes recommended for use
with the system (Table 2-1).
Table 2‑1 Sample Collection Tubes
Collection Tube
Description
Test
Greiner Bio-One Vacuette tube
(Greiner #454322)
Partial fill, 2 mL, 3.2%
sodium citrate. Blue top
Aspirin,
PRUTest,
IIb/IIIa (only for use with ReoPro –
abciximab
)
Greiner Bio-One High Altitude
Vacuette Tube
(Greiner #454323)
Partial fill, 2 mL, 3.2%
sodium citrate. Blue top
Aspirin,
PRUTest,
IIb/IIIa (only for use with ReoPro –
abciximab
)
Greiner Bio-One Vacuette tube
(Greiner #454244)
Partial fill, 3 mL lithium
heparin. Green top
IIb/IIIa - for use with Integrilin (
eptifibatide
) or ReoPro
(
abciximab
)
Nipro
(NP-CW0185-1363080)
1.8mL citrate tube
Aspirin,
PRUTest,
NOTE: The use of sample collection tubes other than those listed may adversely affect test results.
2.4
Quality Control
Accumetrics rigorously tests each VerifyNow System to ensure it meets performance standards. In
addition, the system supports several types of quality controls to verify continued performance during
use.
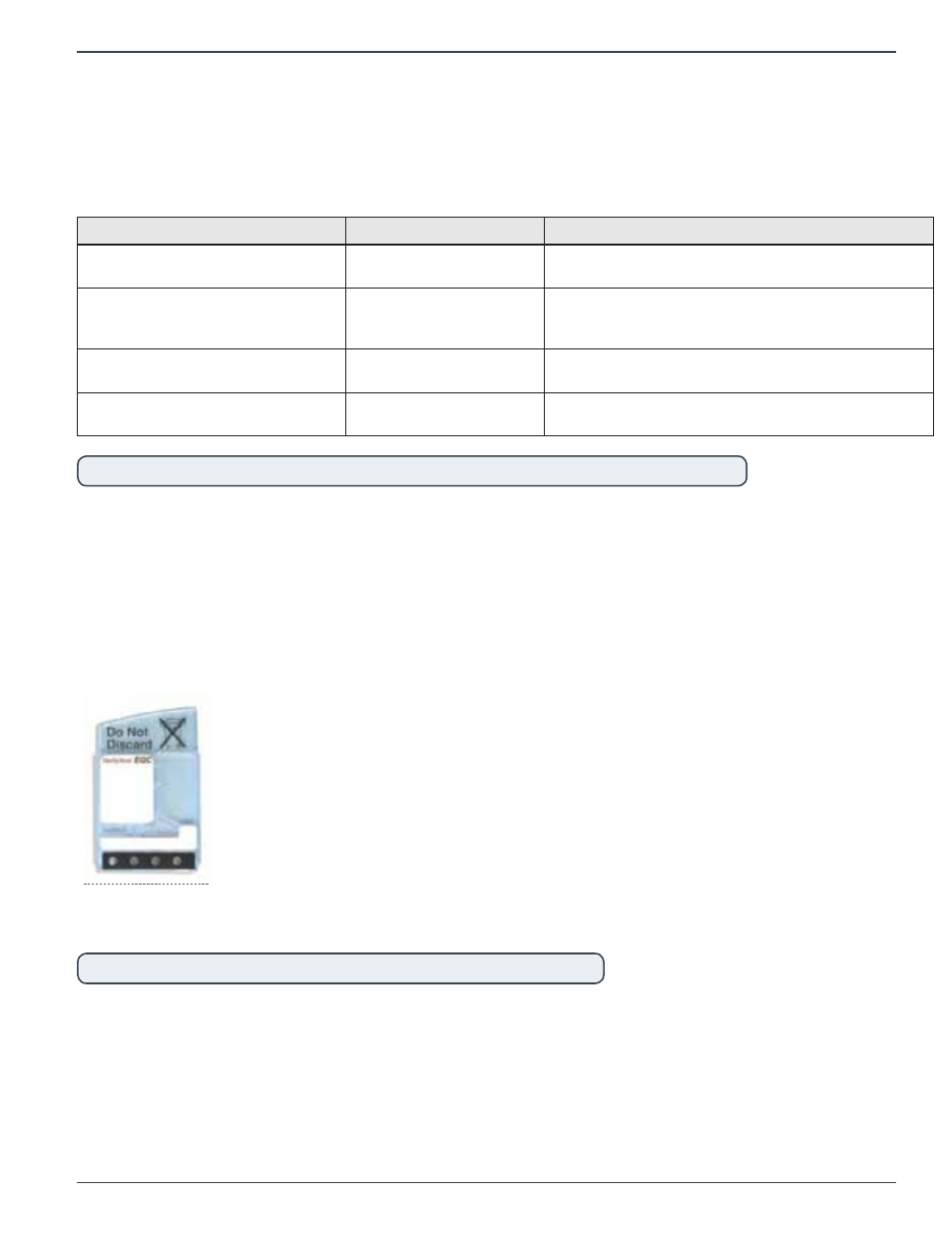
2.4.1
EQC Device and Storage Slot
The Electronic Quality Control (EQC) is the primary quality control mechanism for the VerifyNow
instrument. It consists of a re-usable device that is inserted by the operator into the test port and is
used to perform a comprehensive testing routine that confirms instrument performance.
Figure 2‑6 EQC Device
When not in use, the EQC device is kept in a storage bay on the right side of the instrument.
NOTE: If the EQC device is lost or damaged, the instrument will be inoperable.
