ITC VerifyNow System Platelet Reactivity Test User Manual
Page 14
Overview of System Components - 2
Page 10
14439.C 03/2013
VerifyNow System User Manual |
USA
2.1.8
Printer (Accessory)
The printer is an optional accessory to the instrument. The printer enables an operator to print test
results, QC results, and instrument usage statistics. See section 8.8 for instructions on enabling the
printer.
2.2
Test Device
The VerifyNow System uses disposable test devices to conduct the test. There are three types of test
devices relating to the appropriate platelet function test: Aspirin,
PRUTest, and GP IIb/IIIa inhibitors
(specifically for monitoring abciximab or eptifibatide). Test devices are individually sealed in foil pouches
and may be used until the expiration date printed on each kit box and on the foil pouch.
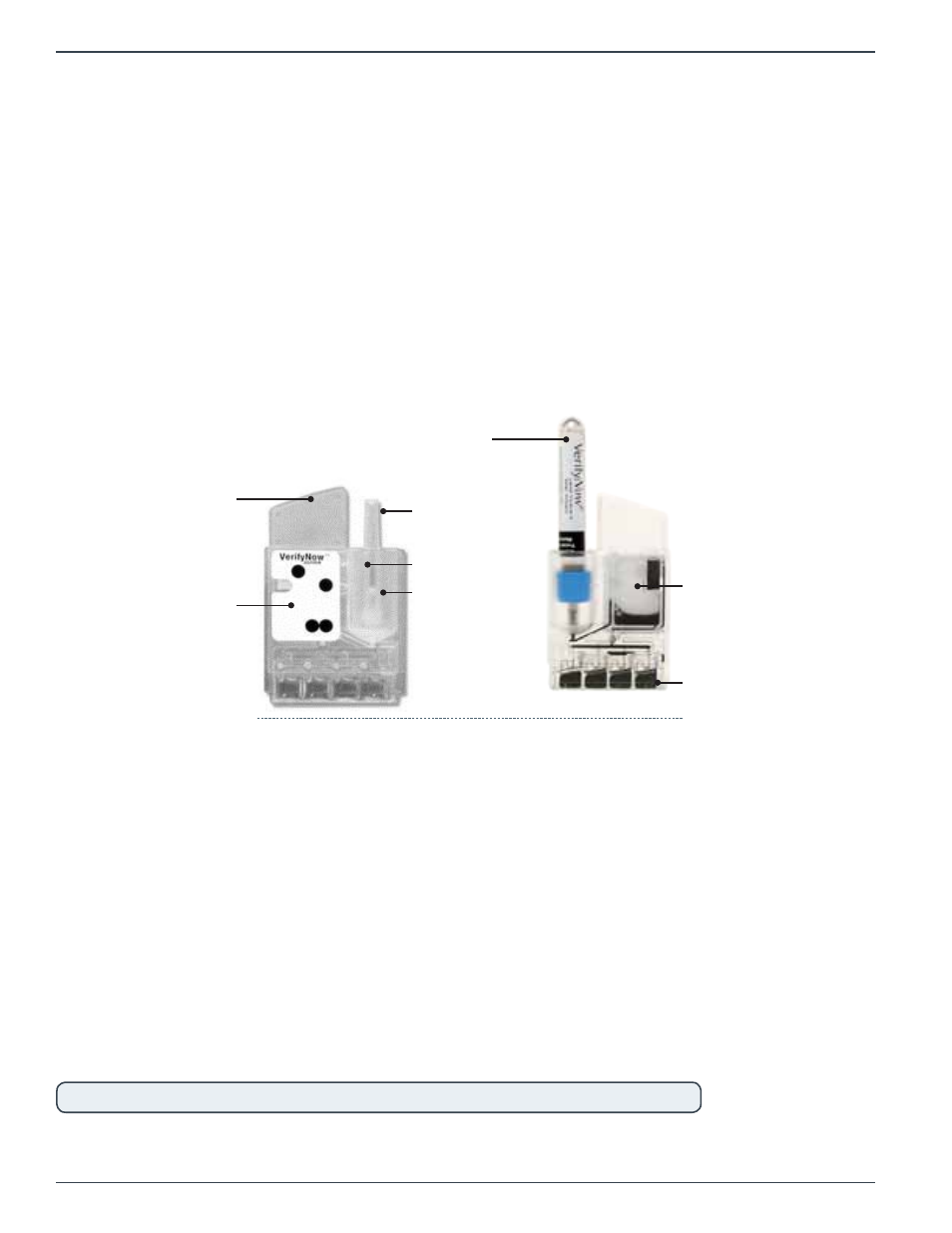
Each single-use test device consists of a sample well, staging well, and four detection wells (Figure
2-5). The instrument automatically draws whole blood into the test device from a sample collection
tube in the sample well. It then heats the blood to 37°C for a period of time specific to each test, and
proceeds with the analysis of the blood in the detection wells.
Figure 2‑5 Test Device
Sample or
WQC Tube
Sample
Well
Detection
Wells
Spot Code
Needle
Sheath
Staging
Well
Finger
Grip
The detection wells of a test device contain a lyophilized preparation of human fibrinogen-coated beads
and platelet agonist. Fibrinogen-coated beads bind to available platelet receptors in the blood sample.
When the activated platelets are exposed to the fibrinogen-coated beads, agglutination occurs in
proportion to the number of available platelet receptors. The instrument measures this agglutination as
an increase in light transmittance through the detection wells.
2.2.1
Spot Code
Each test device is labeled with a spot code. Upon insertion of the test device into the test device
port, the instrument automatically scans the spot code and determines which test is being performed,
whether the particular lot has been used previously, and if the lot expiration date has been reached.
Test devices are calibrated at the factory. No additional calibration is required by the user. Information
about calibration and the device expiration date is contained in the bar code on the pouch of each test
device. The bar code must be scanned whenever a new lot of test devices is to be used.
NOTE: Handle test devices as biohazardous material. Dispose of them in an appropriate manner.
