Poor combustion — 37, Figure 5-6, Combustion capsule with adhesive tape seal — 37 – Parr Instrument 6300 User Manual
Page 37: Perating, Nstructions
O
PERATING
I
NSTRUCTIONS
6300
5
w w w . p a r r i n s t . c o m
37
Note:
Tape should always be stored in a sealed container to
minimize changes in its moisture and solvent content
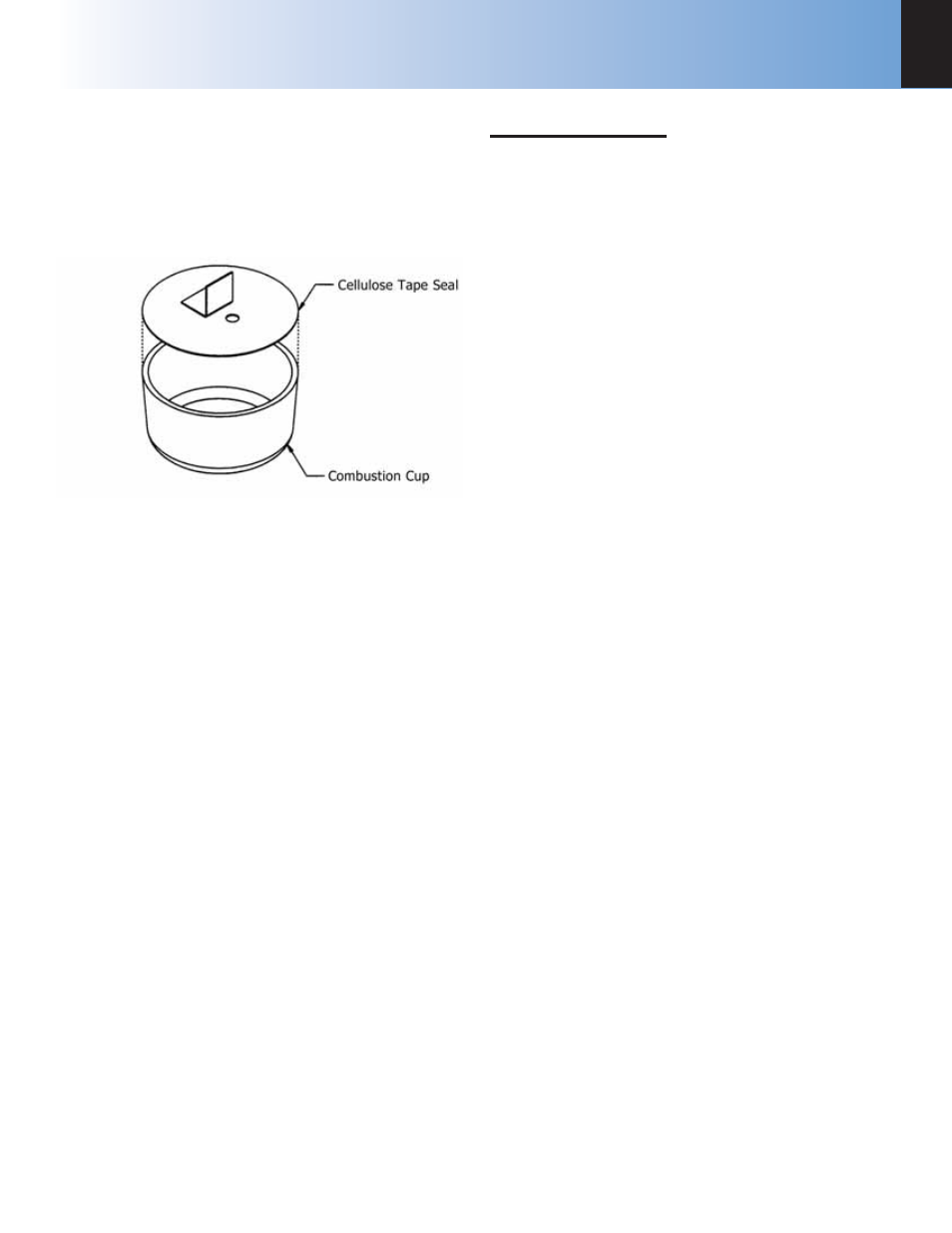
Figure 5-6
Combustion Capsule with Adhesive Tape Seal
Use the following procedure when filling and handling
any of these tape-sealed sample holders:
Weigh the empty cup or capsule; then cover the top
with tape, trim with a knife and press the trimmed edge
firmly against the metal rim. Also cut and attach a small
flag to the disc (see Figure 5-7).
Puncture the tape at a point below the flag, then re-
•
weigh the empty cup with its tape cover.
Add the sample with a hypodermic syringe; close
•
the opening with the flag and re-weigh the filled
cup.
Set the cup in the capsule holder and arrange the
•
auxiliary fuse so that it touches the center of the
tape disc.
Just before starting the test, prick the disc with a
•
sharp needle to make a small opening which is
needed to prevent collapse of the disc when pres-
sure is applied.
Fill the bomb with the usual oxygen charging pres-
•
sure.
The calorimeter will fire the bomb and complete the
•
test in the usual manner.
Volatile samples are defined as one with an initial boil-
ing point below 180ºC per ASTM D-2.
Low volatile samples with a high water content, such
as urine or blood, can be burned in an open capsule by
absorbing the liquid on filter paper pulp or by adding a
combustion aid, such as ethylene glycol.
P
OOR
C
OMBUSTION
Because of the difference in combustion characteristics
of the many different materials which may be burned in
an oxygen bomb, it is difficult to give specific directions
which will assure complete combustions for all samples.
The following fundamental conditions should be con-
sidered when burning samples:
Some part of the sample must be heated to its igni-
•
tion temperature to start the combustion and, in
burning, it must liberate sufficient heat to support its
own combustion regardless of the chilling effect of
the adjacent metal parts.
The combustion must produce sufficient turbulence
•
within the bomb to bring oxygen into the fuel cup
for burning the last traces of the sample.
Loose or powdery condition of the sample which
•
will permit unburned particles to be ejected during a
violent combustion.
The use of a sample containing coarse particles
•
which will not burn readily. Coal particles which
are too large to pass a 60 mesh screen may not burn
completely.
The use of a sample pellet which has been made too
•
hard or too soft. Either condition can cause spalling
and the ejection of unburned fragments.
Insufficient space between the combustion cup and
•
the bottom of the bomb. The bottom of the cup
should always be at least one-half inch above the
bottom of the bomb or above the liquid level in the
bomb to prevent thermal quenching.
Excessive moisture or non-combustible material
•
in the sample. If the moisture, ash and other non
combustible material in the sample amounts to ap-
proximately 20% or more of the charge, it may be
difficult to obtain complete combustion. This condi-
tion can be remedied by adding a small amount of
benzoic acid or other combustion aid.
