Metrohm 765 Dosimat User Manual
Page 19
2.2 Modes
765 Dosimat
15
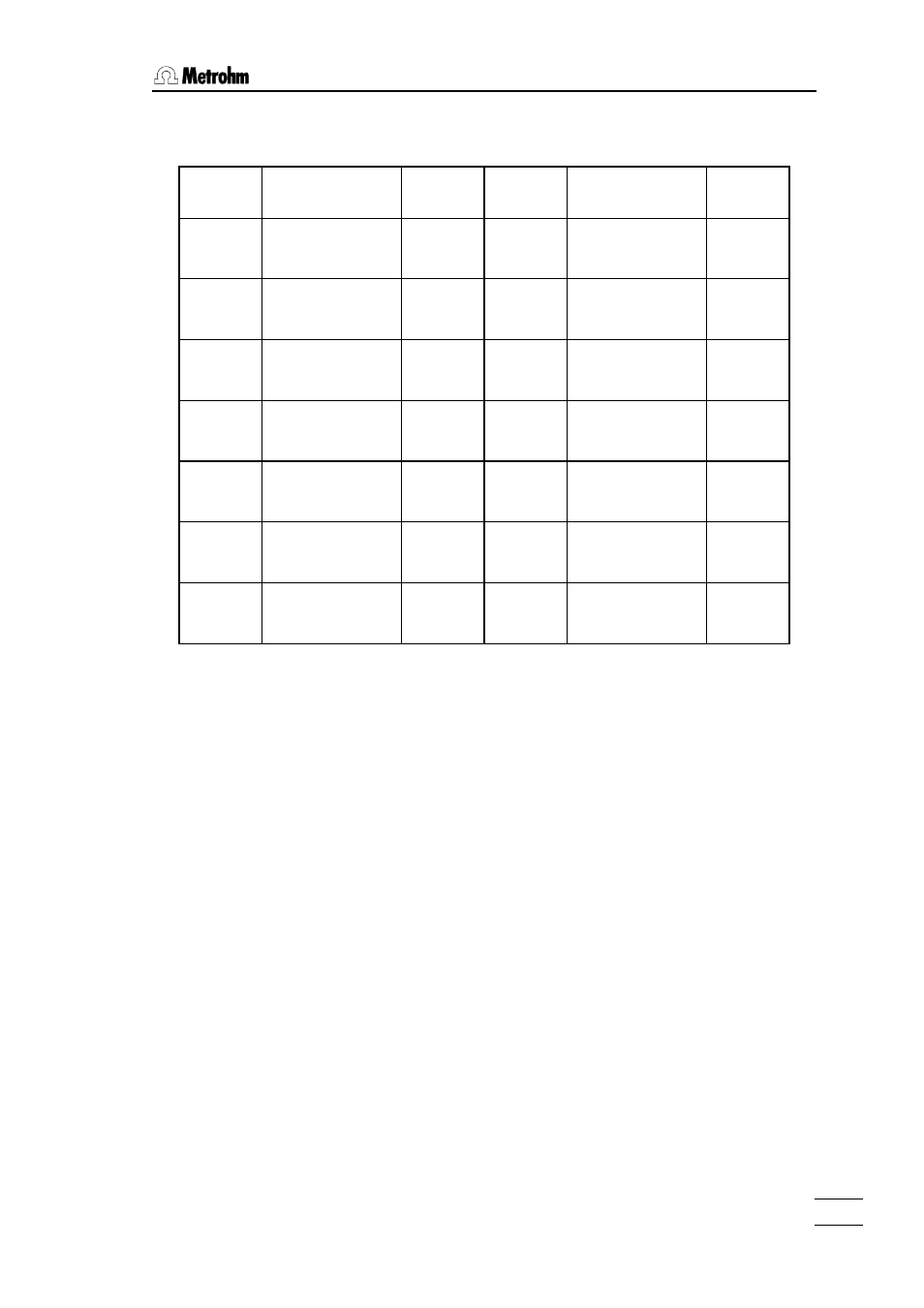
The following table shows factors for the most common ionic standards:
Cation Standard
prepared from:
Factor f
Anion
Standard
prepared from:
Factor f
Na
+
NaCl
NaNO
3
0.39339
0.27050
F
-
NaF
0.45245
K
+
KCl
KNO
3
0.52441
0.38670
Cl
-
NaCl
KCl
0.60666
0.47550
Ca
2+
CaCl
2
0.36111
Br
-
NaBr·2H
2
O
KBr
0.57514
0.67141
Ba
2+
BaCl
2
·2H
2
O
Ba(NO
3
)
2
0.56222
0.52550
I
-
KI
0.76444
Cu
2+
Cu(ClO
4
)
2
Cu(NO
3
)
2
·6H
2
O
0.24214
0.21494
SO
4
2-
K
2
SO
4
0.55087
Pb
2+
Pb(ClO
4
)
2
·3H
2
O
Pb(NO
3
)
2
0.45028
0.62557
NO
3
-
NaNO
3
KNO
3
0.72950
0.61319
PO
4
3-
Na
2
HPO
4
·12H
2
O
Na
3
PO
4
·12H
2
O
0.26519
0.24985
The factor f as correction for substances with admixtures
e.g. water of crystallization, impurities, moisture.
The factor f as correction for the volume contraction
For the amount-of-substance concentration c (units mol/L and mmol/L) and the mass
concentration ρ (units g/L and mg/L), the concentration is referred to the volume of the
solution.
c
i
= n
i
/V resp. ρi= mi/V
where n
i
amount of substance i
m
i
mass of substance i
V
volume of the solution
- 915 KF Ti-Touch (382 pages)
- 800 Dosino (53 pages)
- 767 Calibrated Reference (23 pages)
- 940 Professional IC Vario ONE/SeS/Prep 2 (54 pages)
- 754 Dialysis Unit (49 pages)
- 815 Robotic Soliprep for LC (76 pages)
- Vision Manual (207 pages)
- tiamo 2.1 Manual (1532 pages)
- 825 Lab Link (37 pages)
- 808 Titrando (70 pages)
- 902 Titrando (52 pages)
- 756 KF Coulometer (163 pages)
- 756 KF Coulometer (162 pages)
- 940 Professional IC Vario ONE/LPG (98 pages)
- 850 Professional IC Anion MCS Prep 3 (154 pages)
- 850 Professional IC Anion MCS Prep 3 (152 pages)
- 904 Titrando (58 pages)
- 850 Professional IC Anion MSM-HC MCS Prep 2 (150 pages)
- 930 Compact IC Flex Oven/ChS/Deg (47 pages)
- 872 Extension Module Liquid handling (64 pages)
- 814 USB Sample Processor (90 pages)
- 814 USB Sample Processor (91 pages)
- 940 Professional IC Vario (43 pages)
- Vision – Tutorial (40 pages)
- 799 GPT Titrino (242 pages)
- 889 IC Sample Center (68 pages)
- 761 Compact IC (228 pages)
- 851 Titrando (100 pages)
- 748 DH Sample Changer (32 pages)
- 940 Professional IC Vario ONE/SeS/HPG (51 pages)
- 896 Professional Detector – Amperometry (62 pages)
- 877 Titrino plus (139 pages)
- 881 Compact IC pro – Anion (129 pages)
- 940 Professional IC Vario ONE/ChS/HPG (112 pages)
- 930 Compact IC Flex Deg (41 pages)
- 840 PC Control 5.0 / Touch Control (351 pages)
- 940 Professional IC Vario ONE/Prep 1 (45 pages)
- 776 Dosimat (42 pages)
- 717 Sample Changer (36 pages)
- 815 Robotic USB Sample Processor XL (113 pages)
- 815 Robotic USB Sample Processor XL (114 pages)
- 940 Professional IC Vario ONE/SeS/PP (126 pages)
- 838 Advanced Sample Processor Installation Instructions (109 pages)
- 700 Dosino (55 pages)
- 719 S Titrino (152 pages)
