Memmert CELSIUS 10 FDA User Manual
Page 51
51
CELSIUS 10 FDA-Edition
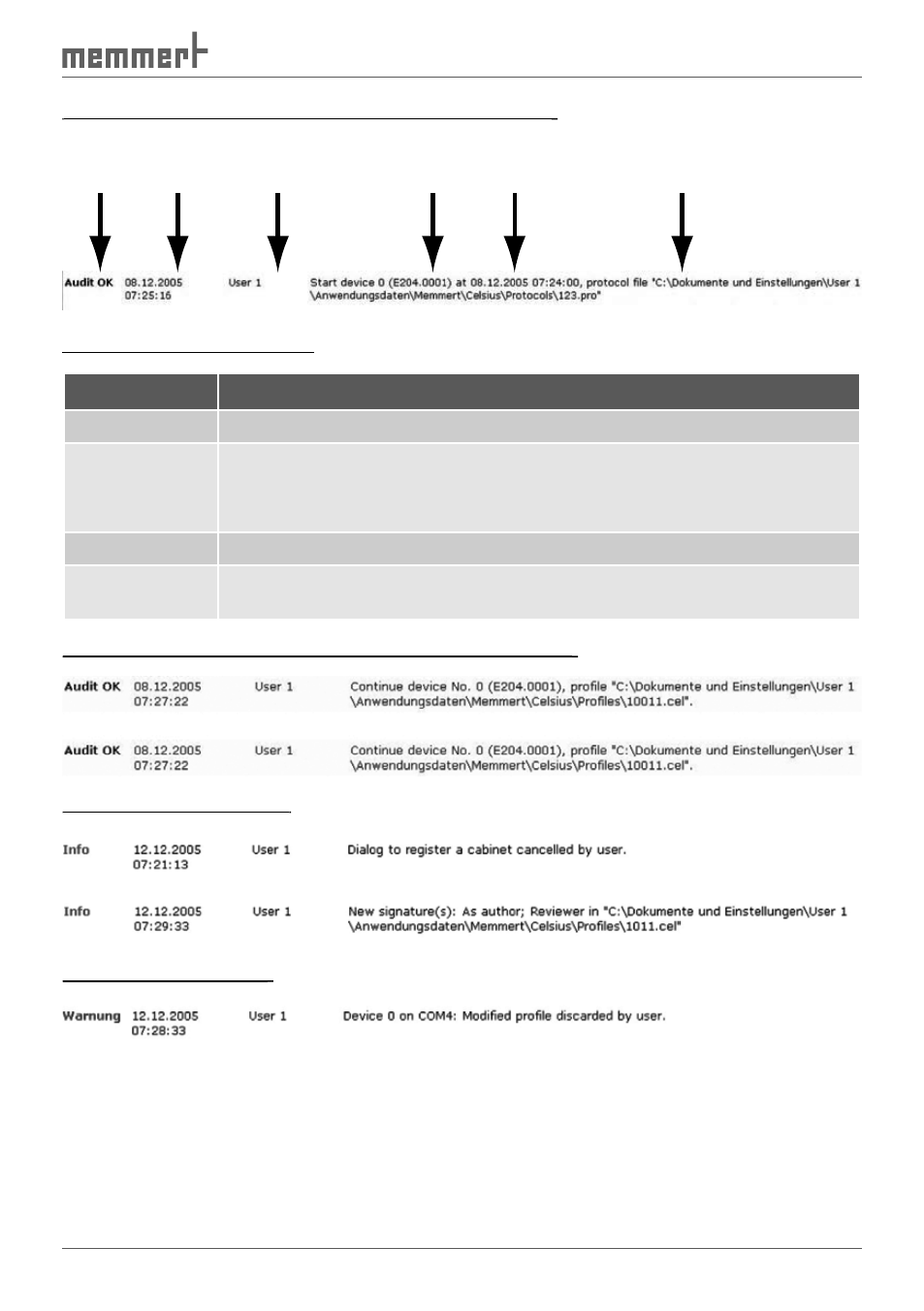
Typical arrangement of an entry in the audit trail file:
Typical arrangement of an entry in the audit trail file:
Type Time stamp
User
name
Device
adress
Device
number
Filename
Forms of audit trail entries:
Report type
Description
Audit OK
General audit trail entries, e.g. during storing
OK
Fehler
Audit trail entries of authority tests:
User authorized for this action
User not authorized for this action
Info
Notes, e.g. on electronic signing of a profile or protocol file.
Warnung
Warnings of certain (uncritical) user activities, e.g. that an alteration of
the profile file has not been saved.
Examples of entries during saving and programme run:
Examples of entries during saving and programme run:
Examples of info entries:
Examples of info entries:
Example of warnings:
Example of warnings:
