Solvent data, Appendix – Heidolph Hei-VAP Precision User Manual
Page 57
Appendix
Appendix
112
113
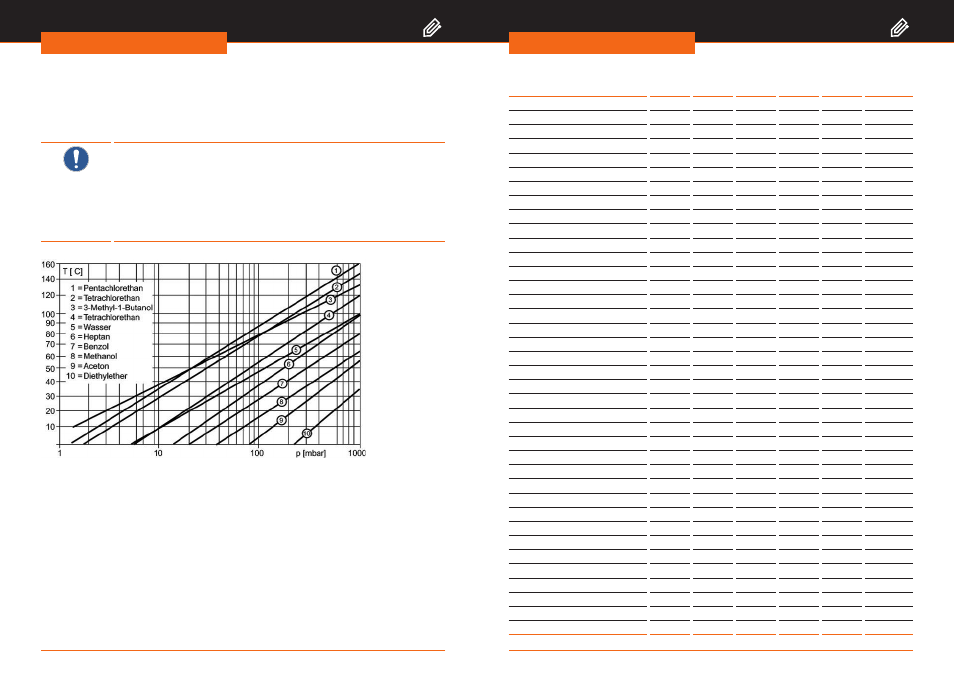
Solvent data
The graph shows the relationship between the pressure and vapor temperature of a
selection of solvents.
Torr to mbar conversion: [mmHg] ≈ 3/4 [mbar]
The temperature difference between the vapor temperature and the
cooling medium should be at 20 K to result in sufficient condensation.
The temperature difference between the heating bath and vapor
temperature should be at 20 K to reach a sufficient Distillation rate (dT).
i.e.: Set a vacuum for a vapor point at 40 °C, set the heating bath
temperature at 60 °C.
Figure 11-1: Graph
Solvents
Total
formula
MW
[g/mol]
Vapor
point [°C]
ΔHvap
[J/g]
Vacuum for a vapor
point at 40 °C
[mbar]
[mm(Hg)]
Acetone
C
3
H
6
O
58,08
56,5
550
556
387
Acetonitrile
C
2
H
3
N
41,05
81,8
833
230
173
Benzene
C
6
H
6
78,11
80,1
549
236
177
n-butanol (butyl alcohol)
C
4
H
10
O
74,12
117,5
619
25
19
tert.-butanol (tert-butyl alcohol)
C
4
H
10
O
74,12
82,9
588
130
98
2-Butanone (methyl ethyl ketone)
C
4
H
8
O
72,11
79,6
473
243
182
Chlorobenzene
C
6
H
5
CI
112,60
132,2
375
36
27
Cyclohexane
C
6
H
12
84,16
80,7
389
235
176
1.2 Dichloroethane
C
2
H
4
CI
2
98,96
82,4
336
210
158
1,2 Dichloroethylene (cis)
C
2
H
2
CI
2
96,94
59,0
320
479
134
1,2 Dichloroethylene (trans)
C
2
H
2
CI
2
96,94
47,8
313
751
563
Dichloromethane (methylene chloride)
CH
2
CI
2
84,93
40,7
373
atm.
atm.
Diethyl ether
C
4
H
10
O
74,12
34,6
392
atm.
atm.
Diisopropyl ether
C
6
H
14
O
102,20
67,5
318
375
281
Dimethylformamide
C
3
H
7
NO
73,09
153,0
–
11
8
1,4-Dioxane
C
4
H
8
O
2
88,11
101,1
406
107
80
Ethanol
C
2
H
6
O
46,07
78,4
879
175
131
Ethylacetate
C
4
H
8
O
2
88,11
77,1
394
240
180
Heptane
C
7
H
16
85,09
98,4
439
120
90
Hexane
C
6
H
14
86,18
68,7
370
335
251
Methanol
CH
4
O
32,04
64,7
1225
337
253
3-Methyl-1-butanol (Isoamyl alcohol)
C
5
H
12
O
88,15
130,6
593
14
11
Pentachlorinated Ethane
C
2
HC
I5
202,30
160,5
203
13
10
Pentane
C
5
H
12
72,15
36,1
382
atm.
atm.
n-Pentanol (amyl alcohol)
C
5
H
12
O
88,15
137,8
593
11
8
1-Propanol (n-propyl alcohol)
C
3
H
8
O
60,10
97,8
787
67
50
2-Propanol (isopropyl alcohol)
C
3
H
8
O
60,10
82,5
701
137
103
1,1,2,2-Tetrachloroethane
C
2
H
2
CI
4
167,90
145,9
247
20
15
Tetrachloroethylene
C
2
CI
4
165,80
120,8
233
53
40
Tetrachloromethane (carbon tetrachlori-de)
CCI
4
153,80
76,7
225
271
203
Tetrahydrofuran (THF)
C
4
H
8
O
72,11
66,0
–
402
302
Toluene
C
7
H
8
92,14
110,6
425
77
58
1,1,1-Trichloroethane
C
2
H
3
CI
3
133,40
74,1
251
300
225
Trichloroethylene
C
2
HCI
3
131,40
86,7
265
183
137
Trichloromethane (chloroform)
CHCI
3
119,40
61,3
263
474
356
Water
H
2
O
18,02
100,0
2259
72
54
Xylene (isomers mixture)
C
8
H
10
106,20
137–143
390
25
19
Table 11-3: Solvent data
